Issue 37 November 2014
Worcestershire Record | 37 (November 2014) page: 23-24 | Worcestershire Biological Records Centre & Worcestershire Recorders
Dragonflies in Worcestershire 2014
Mike Averill
Following the previous year’s prolonged cold spring right in to May, the weather in 2014 was more like a normal year with gradually increasing temperatures through April and May. Through the year 24 species were seen out of the 28 so far recorded in the county. Nearly all species emerged earlier than last year and a few were actually earlier than in the 2008-12 period, namely the Common Club-tail Gomphus vulgatissimus and Scarce Chaser Libellula fulva. It’s worth noting that 13 of our Dragonflies and three of our Damselflies emerge early in spring as a synchronised event. These are usually species that have at least a two year cycle but delay emergence through the previous Autumn and wait until the following year to enable them to emerge early in the next April/May. All the early synchronised species are very susceptible to poor weather and can easily vary their first dates by as much as three weeks.
As the year progressed in to 2014, the weather was occasionally fine and memories of the summer are likely to be good with long spells of fine weather (16 dry days in June, 23 in July, 11 in August and 23 in September), yet as a year it was very wet and September was the only month with below average rainfall. This continuation of regular rain was ideal for dragonflies, wet enough to keep pools full yet with long dry sunny spells allowing the adults to fly regularly. This is good weather for aquatic insects but perhaps not so good for butterflies and bees.
Recording transects are a really useful way to get a meaningful trend in dragonfly numbers through the year, which can in turn be compared to other years. Taking counts over a measured distance and repeating the visit each month in good weather can give a better idea of numbers where subjective impressions cannot. In the case of the counts on the River Avon at Eckington, most species had an upturn in numbers compared to last year, the only exception being the Red-eyed Damselfly Erythromma najas. This upturn followed a two year decline in 2012 and 2013 for the White-legged Platycnemis pennipes, Blue-tailed Ischnura elegans and Common Blue Enallagma cyathigerum damselflies (01). Species with too small a count to see on the graph like Scarce Chaser, Common Darter Sympetrum striolatumand Large Red Damselfly Pyrrhosoma nymphula also had a slight upturn. Another species that has recovered a little in 2014 after a large decline from 2009 is the Banded Demoiselle Calopteryx splendens. It is not clear why 2009 was such a good year for this species but it did have a very warm Spring followed by long hot spells in the summer. Whatever the reason, the species has not done nearly as well since.
In Croome Park all species were also up this year, recovering from a poor 2013. The Small Red-eyed Damselfly Erythromma viridulum did very well indeed this year exceeding all previous years counts. Elsewhere the Small Red-eyed popped up in various places including Lower Smite Farm, Hanley Swan, Purshull Green Pool, Hartlebury Riverside Pool, several pools at Grimley, Pirton Pool, a few river locations on the Avon near Pershore and one farm pond near Berrow in the very south of the county. This is significant because although this species has been with us since 2006, having arrived in the UK in 1998, it has not really consolidated its position elsewhere before this and it was thought that the westerly spread had slowed up to a halt.
Other dragonflies that are doing well are the Four-spotted Chaser Libellula quadrimaculata and the Beautiful Demoiselle Calopteryx virgo. The Four-spotted chaser seemed to appear in all sorts of locations this year whereas it was confined to just a couple of sites 20 years ago. The Beautiful Demoiselle is another species that has changed its distribution where, 20 years ago it stuck to the westerly tributaries of the Severn it can now be found in small streams throughout Worcestershire and even on the river Cole in the old Vice County area of Birmingham (A separate account from Des Jennings will appear in volume 38 April 2015 issue of Worcestershire Record).
The Scarce Chaser Libellula fulva was quite prominent on its stronghold along the Avon and has in fact spread up to the Warwickshire border this year. It was seen on Hillditch Pool again where it breeds in low numbers and also once again at Hurcott Pool where a mated male was seen.
Another species, also found on our rivers at the same time, the Common Club-tail Gomphus vulgatissimus had another good year at Bewdley where emergence numbers were the third highest in seven years. On the Avon though, there are signs that it is struggling as fewer individuals were seen above Pershore and apparently none were seen in Warwickshire for the second year running. It is hard to know why this is happening as the Scarce Chaser which has a similar life cycle and habitat requirements is doing quite well and so does not suggest there is a water quality issue.
Our regular visitor the Red-veined Darter Sympetrum fonscolombii made an early appearance(23/6) at Pirton Pool but wasn’t seen anywhere else this year.
Normally only seen in the Wyre Forest area, the Golden Ringed dragonfly Cordulegaster boltonii was seen by chance at the Devils Spittleful egg laying in a leakage by a water trough. There is no mistaking this species, when seen ovipositing, as the female prods her abdomen in soft silt like a garden dibber to insert her eggs. This species is normally found on rivulets and seepages, so it was a surprise to find two shed larval cases, this year, along the Severn at Bewdley but this location was just downstream of Dowles Brook so perhaps they had been washed out of there in the January floods.
A late species to emerge, the Migrant Hawker Aeshna mixta, was very evident in September due to the fine weather and it gave the opportunity to watch where they laid their eggs at Hartlebury Common. They choose the Soft Rush Juncus effusus and lay about 10 cms above the water line in to the older browner stems. They may well try other woody vegetation but reject anything hard like brambles and tree species preferring rush where the eggs can be inserted in to the stems to lie in the soft tissue inside(02). Each egg is about 1.7mm long shaped like a long cylinder with an opening called a micropyle at one end. This opening firstly allows sperm to enter the egg for fertilisation and later it is the place where the prolarvae emerges. The egg is laid at an angle downwards in to the plant so that the micropyle is nearest to the exit hole. The recess in the plant tissue is made by a knife like device called the ovipositor which cuts a hole and also carefully inserts the egg in to the slot. The way that the dragonfly exerts enough pressure to punch a hole in to the plant is by forming an arch over the stem between the legs and the end of the abdomen and by flexing the middle abdomen the ovipositor is forced in to the stem (03). Each egg is laid separately within its own hole, the next one being laid about 0.5 cm away so that on average there are 25 eggs per 10 cm. These eggs will remain safe and secure in the stems until the spring when they emerge as prolarvae and drop in to the water below. All hawker dragonflies and all damselflies lay eggs in to plant material like this while the darters, skimmers and chasers lay their cluster of eggs on to plants or directly in to water.
An uncommon Worcestershire dragonfly, the Common Hawker Aeshna juncea was seen twice this year, once at Penny Hill egg laying and once at Hartlebury Common.
The mention of Hartlebury Common twice in an article about dragonflies is a real treat as it is a site that has deteriorated badly over the last 50 years due to drying out of water features. This year was without doubt the best year for dragonflies in recent memory with 18 species recorded there. The key to this was the fact that we have had three wet years in a row leading to permanent standing water in the Rush Pool and the Bog which has enabled species with two and three year cycles to succeed in breeding there. It is a lowland heath site and is the only place in Worcestershire where we can see the classic heathland species like Common Hawker, Emerald Damselfly Lestes sponsa and Four-spotted Chaser together. The only one missing is Black Darter Sympetrum danae so perhaps that one will appear next year as it has been seen at Hartlebury in the past. Other than these heathland species, the fact that 18 species were present through the year made the site quite a spectacle and provides an opportunity to really compare similar species like Scarce Chaser, Broad bodied Chaser Libellula depressa and Four-spotted Chaser.
While the wet weather has helped the situation on Hartlebury Common the chances are that we will return to drier conditions in the coming years and so it is important that the Council and Natural England press ahead with plans to support the water levels on the site during dry spells using a groundwater pump. This will not only benefit dragonflies but all the other aquatic invertebrates and plants that make the site special.
| 01. Eckington dragonfly transect 2009-14. |
| 02. Migrant Hawker egg in plant stem. Mike Averill. |
| 03. Migrant Hawker inserting egg into Juncus effusus stem. Mike Averill |
Worcestershire Record | 37 (November 2014) page: 23-24 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 4 | Worcestershire Biological Records Centre & Worcestershire Recorders
Identification of Scorpion Flies
Mike Averill
Following Martin Mathews excellent Gloucester Mecopterans numbers 1 & 2 reprinted in the Worcestershire Record No 36 there is at last a reliable way to identify the three different species likely to be encountered in Worcestershire. Following recording in 2014 this note confirms that it is easy to identify the males without the need for dissection. All that is needed is an examination of a male or of a good photograph of the underside of the male genital capsule. Thankfully the male usually holds this uppermost giving the group its familiar name. When held like this there are two structures called hypovalves on the surface which are characteristic of the species (see guide 01).
Both Panorpa germanica and P. communis are reasonably common, but P. panorpa is less likely to be found.
Although it is recommended that the genital capsule is the positive way to split the species, the markings do seem different within the three species seen. Panorpa communis usually has the darker black markings with very little additional spotting. P. communis and P. germanica are superficially more similar with paler black markings and more of them. There is fourth species P. vulgaris which may well arrive in the UK and it is similar to P communis but with more dark black spots so watch out for that.
It is hoped that this note will aid the further identification of Scorpionflies in the county.
Reference
Matthews, M. 2014. The Gloucester Mecopteran. Worcestershire Record 36:24-27
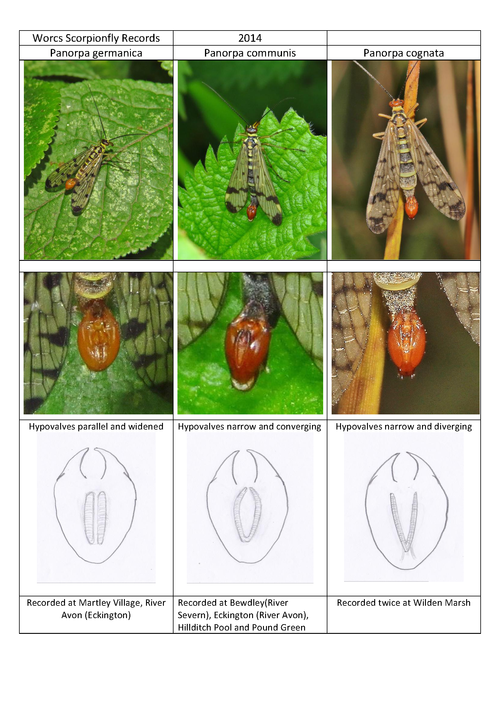
01. Guide to identification of scorpion flies. Mike Averill
Worcestershire Record | 37 (November 2014) page: 4 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 10-11 | Worcestershire Biological Records Centre & Worcestershire Recorders
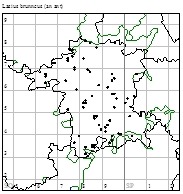
Lasius brunneus (Latreille, 1798). The Tree Ant, Nationally Notable (Na) at Hurcott, Kidderminster
John Bingham & Denise Bingham
In the Worcestershire Record Harry Green (1998) provided a note on the distribution of Lasius brunneus in Worcestershire drawing attention to a paper by Alexander and Taylor (1998) on the Severn Valley as a stronghold for the species. The Thames Valley was the main stronghold for the species until the 1960s when it was reported from the Severn Vale in Gloucestershire and south Worcestershire. Shropshire had one record from Dudmaston Park in 1996. Parkland sites such as Croome Park and Hanbury Park appear to be the typical habitat in Worcestershire. Harry considered that it seemed likely that the species has been under-recorded rather than showing a recent extension of range. More records are now known but the ant is probably still under- recorded.
On 21st April 2014 Denise and I were recording our local patch at Hurcott, Kidderminster when Denise swept up a number of ants from a patch of flowering White Deadnettle Lamium album at grid reference SO848776. I identified the ants as Lasius brunneus but to confirm this I sent an image to Harry and Geoff Trevis, who both who agreed (01). The area around Hurcott has a number of large oak trees in the fields and hedgerows and the land is managed as low intensity horse pasture. Whilst not parkland it has some similarities, so it is perhaps not that surprising that L. brunneus was found here. Why it was swept off L. album is not clear, perhaps an early nectar source or were tiny aphids present on the plant? Searching the large oak trees nearby a few days later failed to reveal any ants, but they do remain well hidden in crevices so perhaps this was to be expected. The hedgerow had many old dead and dying bushes and old wooden fence rails, all possible habitat for ants. I did eventually find the ant in low numbers on a sycamore tree about 0.4 km away to the north at SO847779.
As worker ants are fugitive and rarely seen on the host tree surface perhaps sweeping any flowering plants of L. album in early spring might be a way of providing records of this elusive ant, especially if large oak trees are present nearby. As yet we have yet to see the ant on the nearest old oak to the Hurcott L. album site, but the tree has deep crevices!
Acknowledgements
Thanks to Harry and Geoff for the identification.
References
Green, H. 1998. The brown ant: Lasius brunneus (Latreille). Worcestershire Record 4:12.
Alexander KNA & Taylor A. 1998. The Severn Vale, a national stronghold for Lasius brunneus (Latreille) (Hymenoptera: Formicidae). British Journal for Entomology & Natural History 10:217-219.
Editors note.
Since 1998 Lasius brunneus has been found in many places across Worcestershire almost always on tree trunks in parkland, orchards and elsewhere. Special studies of old orchards for other species have almost always detected Lasius brunneus. I (Harry Green) have swept it off flowering Ramsons Alium ursinum on two occasions in May, once on hawthorn flowers Crataegus monogyna in May and also amongst leaf litter beneath trees on one occasion. These visits to plants other than trees are rarely reported and not understood.
Geoff Trevis is producing a Worcestershire Atlas of Aculeate Hymenoptera which should be printed in 2015. His comments together with a distribution map (02) on Lasius brunneus appear below: compare with the map from the 1998 paper (03).
Lasius brunneus (Latreille, 1798).
Formicidae: Formicinae
Has a curious distribution with two separate populations, one based around the Thames valley and extending north into East Anglia and south into the North Downs and the other in the Severn Valley up to Shropshire. In these areas it is common and not threatened.
Habitat: Generally nests in mature, living trees but has been found in stumps, hedgerows and timber framed buildings.
Flight Period: Queens and males fly in June or early July.
Worcestershire Records: The species is widely distributed across the county with 92 widely distributed records. Nationally this remains a rare species.

01. Lasius brunneus at Hurcott. John Bingham.
02. Lasius brunneus distribution map 2014
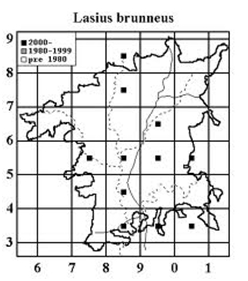
03. Lasius brunneus distribution map 1998
Worcestershire Record | 37 (November 2014) page: 10-11 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 11-12 | Worcestershire Biological Records Centre & Worcestershire Recorders
Mordellistena (Mordellistena) variegata (Fabricius, 1798) (Coleoptera, Mordellidae) at Kidderminster
John Bingham & Denise Bingham
On 24 July 2014 Denise beat a small beetle from a Rowan tree in our garden (01, 02). It was quite well marked and colourful but belonged to a group of tumbling flower beetles Mordellistena, not easy to identify and with some quite rare species. Help was at hand and thanks to Paul Whitehead who identified the beetle from an image sent to him as Mordellistena (Mordellistena) variegata and provided information on distribution. In the note that follows he reports more fully on identifying species in this small group of interesting beetles.
The beetle is widespread but localised in the English midland region extending from lowland river floodplain level to at least 160 m a.s.l. on Bredon Hill, Worcestershire. Host trees include Pedunculate Oak Quercus robur L., Field Maple Acer campestre L. and now Rowan Sorbus aucuparia L. This last is perhaps unsurprising given that M. variegata is a well-known inhabitant of traditional pome fruit orchards in the region.
The Mordellidae or ‘tumbling flower beetles’.
Paul Whitehead
The Mordellidae or ‘tumbling flower beetles’ has always challenged human comprehension. The late Mr A. A. Allen, one of the greatest of the recent British coleopterists described new species of Mordellistena in both 1995 and 1999 neither of which have stood up to subsequent scrutiny. The group is generally well known for the apical abdominal segment being extended to form a so-called pygidium (Gr. pygidion = rump). This is especially conspicuous in the black Mordellistena which are especially speciose in southern Europe and which mostly breed in the rigid stems and rootstocks of Asteraceae, including Artemisia spp. and occasionally those of other groups such as Campanulaceae (e.g. Jasione).
Only three of the 12 British species of Mordellistena are not black viz. the scarce widespread M. neuwaldeggiana (Panzer, 1796) which is more or less uniformly brownish-orange and thus immediately recognisable; M. humeralis (L., 1758) which is variegated black and dull yellow-orange rarely with the pronotum darkened and M. variegata (F., 1798) which (in numerous examples seen) has clear orange vittae running obliquely away from the elytral humeri and the pronotum usually darkened but paler laterally and with the antennae longer. These three species are arboreal as larvae in a wide range of deciduous trees usually in soft delignified wood; M. neuwaldeggiana occurs in traditional orchards. In the English midlands I have numerous records of these species with the exception of M. humeralis which I have not yet seen in Britain. Male M. variegata have diagnostic subcircular last palpal segments whereas these are not dilated in male M. humeralis which also has relatively shorter antennae.
Other genera likely to be encountered in the region are Mordellochroa and Variimorda. Female Mordellochroa abdominalis (F., 1775) are unique and unmistakeable in the British fauna being black with clear red pronota; the males have darker pronota and may be confused with species of Mordellistena and Mordella. Ash (Fraxinus excelsior L.) is a known larval host in Worcestershire. Variimorda villosa (Schrank, 1781) is the only British representative of the genus and may be recognised by the pattern of shimmering bronze hairs forming transverse fasciae on the black elytra. Both of these robust species are arboreal as larvae although V. villosa has a demonstrable preference for Salicaceae in riparian situations. Mordella, a genus of mostly robust black species includes two British species which are either rare or localised; M. holomelaena Apfelbeck, 1914 may occur in the region but I am not aware of modern records.
For anyone wishing to acquaint themselves with the finer details of this group I would recommend perusal of Batten, R., 1986. A review of the British Mordellidae (Coleoptera). Entomologist’s Gazette 37:225-235. They might also consider forming a collection taking no more than a minimal number of specimens. As ‘tumbling flower beetles’ mordellids prefer to visit flowers with exposed nectaries, notably those in Rosaceae and Apiaceae and with records also from flowers of Sycamore Acer pseudoplatanus L. Finally (with a few clear exceptions) do not rely on the internet to inform decisions; many such images are based on misidentifications.

01. Mordellistena variegata at Kidderminster. John Bingham.

02. Mordellistena variegata at Kidderminster. John Bingham.
Worcestershire Record | 37 (November 2014) page: 11-12 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 12-14 | Worcestershire Biological Records Centre & Worcestershire Recorders
Coleoptera of note in the Kidderminster area 2014
Alan Brown
I spent the season occasionally visiting a couple of my local haunts, Springfield Park (SO87) and Devil’s Spittleful Nature Reserve (SO87), but my main focus concentrated on a 400 metre stretch of River Severn bankside (SO77) that runs alongside Bewdley town centre, downstream towards Stourport. All observations were made at night with a headband torch.
01. Aegialia sabuleti Scarabaeidae (Nationally scarce) 3/5/2014
A riparian species of scarab beetle found on bare river sandbanks on rivers and streams where it feeds on decaying vegetable matter. This species is very local in the south with most records coming from northern England. Found active at night alongside the river in Bewdley.
02. Dorytomus tremulae Curculionidae (Nationally scarce) 22/4/2014
A species of catkin weevil restricted to feeding on Aspen trees, the adults feed on leaves but the larvae develop in the catkins. I found this one active at night on a mature Aspen trunk at Springfield Park, Kidderminster. Also seen on Aspen alongside the River Severn at Bewdley.
03. Dorytomus tortrix Curculionidae (Local) 23/4/2014
Another species of catkin weevil linked to Aspen, I found these in large numbers on Aspen trees in Kidderminster and along the River Severn in Bewdley on 3/5/2014.
04. Xyleborus saxesenii Scolytidae (Local) 3/5/2014
A rather local bark beetle in Worcestershire, I found a number of these on decaying bark on a dead plum tree alongside the river Severn, Bewdley, active at night.
05. Stereocorynes truncorum Curculionidae (Nationally rare) 22/5/2014
A scarce saproxylic weevil that feeds on dead wood and is nocturnal and seldom seen. I found a number of these on an ancient hollow Sycamore tree at night in shaded woodland at the Devil’s Spittleful NR but it is usually linked to hollow oak trees.
Enicmus rugosus Latridiidae. (Nationally scarce) 22/5/2014
I found two of these small scavenger beetles on a bracket fungus on a dead standing oak tree at the Devil’s Spittleful NR. The species feeds on various fungi and slime moulds.
06. Platystomos albinus Anthribidae.(Nationally scarce) 7/6/2014
A fungus weevil from the edge of the River Severn at Bewdley. This is an unusual record as it was found feeding on mouldy wood on a decaying ash bough infested with Cramp ball fungus which is usually a habitat of its sister species Platyrhinus resinosus. Perhaps there is some competition between these two species. Usually this species is linked to fungoid oak. In 2012 John Bingham recorded one on oak on the Shropshire side of the Wyre Forest.
07. Clitostethus arcuatus Coccinellidae (RDB1) 23/5/2014
These species was seen breeding again at Crossley Park and Hurcott Wood and I was delighted to find it on Greater Celandine Chelidonium majus at the base of an old hawthorn tree alongside the River Severn at Bewdley and on honeysuckle in woodland alongside the Devil’s Spittleful NR. Definitely spreading and good to see it doing so well (Whitehead & Brown 2012).
08. Cyanostolus aeneus Monotomidae (Nationally rare) 10/7/2014
A predatory riparian species. I found two of these on the wet section of a log jutting out of shallow water on the River Severn at Bewdley. The log was too old to make a positive identification of the type of wood, but when disturbed the one specimen took flight; I don’t think night flight has been recorded in this species before.
09. Bracteon litorale Carabidae (Nationally scarce) 27/7/2014
I found three specimens of this predatory ground beetle active at night on a bare sandy river bank at Bewdley. This species is usually described as diurnal so this was also an interesting observation.
10. Amara praetermissa Carabidae (Nationally scarce) 27/7/2014
A seed-feeding ground beetle. Previously recorded at the Devil’s Spittleful heath, I recorded this one in 2011 at Hartlebury Common in sandy heathland. I was surprised to also come across this species on dry areas of sand banks along the River Severn at Bewdley. It was regularly seen there during the summer months.
11. Dyschirius aeneus Carabidae (Local) 4/7/2014
This is the black colour variation of the species; there is also a brassy version. A predatory ground beetle linked to species of Bledius (Col., Staphylinidae). I found these on bare sand banks along the River Severn at Bewdley.
Acknowledgements
Many thanks to John Meiklejohn for his help especially in my early studies. And a big thank you to Paul Whitehead for his help in identifying a lot of these species which has been a big help to me.
Reference
Whitehead, P.F. & Brown, A. 2012. Clitostethus arcuatus (Rossi, 1794) (Col., Coccinellidae) breeding in the Kidderminster area of Worcestershire: overwintering strategies and breeding biology. Worcestershire Record 33:20-22

01. Aegialia sabuleti

02. Dorytomus tremulae

03. Dorytomus tortrix.

04. Xyleborus saxesenni

05. Stereocorynes truncorum

06. Platystomos albinus

07. Clitostethus arcuatus in Bewdley.

08. Rhizophagus aeneus

09. Bracteon literale

10. Amara praetermissa

11. Dyschirius aeneus
Worcestershire Record | 37 (November 2014) page: 12-14 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 15-16 | Worcestershire Biological Records Centre & Worcestershire Recorders
Hornet Rove beetle Velleius dilatatus (F., 1787) (Col., Staphylinidae) at Kidderminster, Worcestershire
Alan Brown
On the 15 June 2014 three specimens of the endangered Hornet Rove Beetle Velleius dilatatus (F.) were found at night at an oak sap-run in woodland alongside the Devil’s Spittleful Nature Reserve, Kidderminster.
Distribution.
Historically this species had only been recorded from the New Forest, Windsor Forest and Moccas Park, but recently it has been recorded further afield at Dartmoor Deer Park, Epping Forest, Norfolk, Nottinghamshire and Lincolnshire.
Habitat
A sap-run was located on a mature Pedunculate Oak Quercus robur L. (01 & 02) just inside the fringe of a patch of mixed ancient woodland most of which is privately owned. However, a public path leading to the heath through this area allows access to some of the ancient trees, mainly oak, but also birch, Sycamore and lime. A vigil was arranged to see what species turned up at night on the sap-run with interesting results. Two notable species, Cryptarcha strigata (F.) and Cryptarcha undata (Ol.) appeared in numbers with an Epuraea species and a number of Soronia grisea (L.). However, my attention was drawn by a large black rove beetle and I was convinced that it matched the description of Velleius dilatatus, an endangered species. Photographs (03, 04, 05 & 06) were taken and this identification was confirmed by Paul Whitehead who also noted that the sap-run was possibly a Cossus flux but this could not be confirmed.
Behaviour
At least three different individual Hornet Rove Beetles were observed over the following seven nights, sometimes all three together. The white light on my headband torch disturbed them too much to observe any behaviour so I switched to a red light which they were not able to detect. Each night the Hornet Rove Beetles appeared at the sap-run within an hour of it becoming dark and remained there for most of the night. All three were males and always kept some distance between each other at different points around the sap-run. Eventually one male started investigating the sap and at first I thought he was ingesting the sap but it soon became obvious that he was looking for something in it. He then extracted what looked like a white larva, possibly a fly larva although this could not be verified. Later a second beetle retrieved what looked like a fly pupa (07 & 08) but again I could not make a definite identification. However, one larva I did find in the sap was positively identified by Paul Whitehead as Cryptarcha.sp. A daily night time temperature taken at the base of the tree averaged a mild 16° Celsius. Random checks of the sap-run were made during daylight hours but no Velleius dilatatus were seen. After a week the sap-run dried up and that was the end of my observations.
Notes
The Hornet Rove Beetle is an interesting species that spends almost its whole life living in hornet’s nests and is therefore dependent on the success of its host. There are various theories as to what it feeds on, some literature suggests it is parasitic on fly maggots that infest the hornet’s nest or that it feeds on hornet detritus and even on dead or dying hornets. In recent years however the range of the hornet has spread slowly northwards and likewise the range of this rove beetle. Although there is no doubt that this species does most of its feeding within the nest there are obvious indications here that when food is scarce the beetle is drawn to sap-runs to seek alternative prey. It clearly responds well to olefactory cues and can fly well and link the odour of sap to its preferred food. This find, which is a first for Worcestershire, would also seem to indicate that Velleius dilatatus may become more widespread in the future.

01. Oak tree on which sap run was found.

02. Sap-run on oak tree

03. Velleius dilatatus

04. Velleius dilatatus

05. Velleius dilatatus

06. Velleius dilatatus

07. Velleius dilatatus with fly pupa

08. Velleius dilatatus with fly pupa
Worcestershire Record | 37 (November 2014) page: 15-16 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 16-18 | Worcestershire Biological Records Centre & Worcestershire Recorders
Stenelmis canaliculata (Gyllenhal, 1808) (Col., Elmidae) and other aquatic Coleoptera from the Kidderminster and Bewdley areas of Worcestershire
Alan Brown.
On the 24 August, 2014 a single specimen of our largest Riffle Beetle Stenelmis canaliculata was found on a sandbank alongside the River Severn at Bewdley (01 & 02). This is the first Worcestershire record and its identity was confirmed by Paul Whitehead. The current national status is RDB2.
Description: About 5mm long, sculptured on the elytra with four prominent ridges, two of which extend the full length of the elytra, the two inner ridges extending to about halfway down. The pronotum is square with a visible channel down the centre. The tips of the tarsi and apical segments of antennae are red.
Distribution: Recorded from 12 rivers in the last 20 years. First discovered at Lake Windemere in Cumbria, this species has a scattered distribution mainly in fast-flowing rivers in Herefordshire, Devon, Cornwall, Nottinghamshire and Bedfordshire. There is a 1996 record apparently from the Kennet and Avon Canal near Bath North Somerset and a more recent one from the River Findhorn at Moray, Scotland.
History: This species was not added to the British list until 1960 when it was found on a wave-washed shingle bank on Lake Windermere. Since then, records have predominantly come from clean, fast-flowing streams and rivers with gravelly or stony bottoms. Like some other species of elmid it needs well-oxygenated water free of pollution making it a very good indicator of water purity. The beetle itself extracts oxygen from the water and does not need to surface except for a brief period of flight shortly after emergence from its pupa. It is thought to feed on algae attached to stones and prefers deep water.
Notes: Not much is known about this beetle’s life cycle. I found the beetle, in this case an adult female, on sandy ground close to the water’s edge at night. At the time I was looking for ground beetles so I was quite surprised to find it there. It was on a cold autumn evening and the beetle seemed to be moving away from the water. I have seen other elmids above water level so perhaps this was not such an unusual event. With my headband torch I had no difficulty in picking it out despite its small size.
Other Notable Finds
Macronychus quadrituberculatus Müller, 1806. Elmidae: Nationally rare. River Severn, Bewdley, 10 August 2014. The River Severn is a well-known stronghold for this species and I saw them on most of the pieces of decaying wood in shallow water and also occasionally on sandbanks close to the water’s edge. They are quite colourful and unusual with long legs (03).
Pomatinus substriatus (Müller, 1806). Elmidae: Nationally rare. Kidderminster 22 April 2014. Ironically, I recorded this species not in the River Severn, but in the Kidderminster to Cookley section of the Staffordshire and Worcestershire Canal running alongside Springfield Park. It was spotted at night apparently feeding on algae growing on the canal’s concrete wall about two inches below the water surface. Three other beetles from bare mud alongside a stream running through the park were identified as the dryopids Dryops ernesti des Gozis, 1886 by Paul Whitehead.
Hydrochus elongatus (Schaller, 1783). Hydrophilidae: Nationally scarce. Kidderminster 23 April 2014. A Water Scavenger Beetle found in good numbers in a shaded willow carr bog pool at Springfield Park. The pool itself is a seasonal one tending to dry up in the summertime, but for the last two years has remained full. The pool contains decaying leaves and wood with some mosses. The beetle was easily found at night walking underneath the surface tension of the water and could be scooped up without using a net (04).
Helophorus dorsalis (Marsham, 1802). Hydrophilidae: Nationally scarce Kidderminster 22 April 2010. A Water Scavenger Beetle found during my Ground Beetle survey. This species was found in the same shaded willow carr bog pool at Springfield Park as Hydrochus elongatus and found in the same way. Good numbers were found at night on the water surface.
Acknowledgements
Unfortunately, the 2014 season was to be my last recording for the Worcestershire Biological Records Centre in Worcestershire as I moved elsewhere at the end of the year. It was a memorable six years. Within a two mile radius of Kidderminster, some 101 nationally scarce species of Coleoptera were found together with nine RDB species. There are undoubtedly many more there still to be found. I would like to thank John Meiklejohn, Paul Whitehead, Harry Green, Simon Wood and Rosemary Winnall for the help they gave me during this survey. I am grateful to Paul Whitehead for his constructive assistance in the preparation of this short paper; any factual errors are mine alone. Good luck to everyone for 2015.

01. Stenelmis canaliculata River Severn, Bewdley, 10 August 2014. Alan Brown

02. Stenelmis canaliculata River Severn, Bewdley, 10 August 2014. Alan Brown

03. Macronychus quadrituberculatus River Severn, Bewdley, 10 July 2014. Alan Brown

04. Hydrochus elongates Kidderminster 22 April 2014. Alan Brown
Worcestershire Record | 37 (November 2014) page: 16-18 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 20-21 | Worcestershire Biological Records Centre & Worcestershire Recorders
The Elm Leaf Hopper Iassus scutellaris (Fieber, 1886) at large in Worcestershire
Gary Farmer
The leaf hopper Iassus lanio will be a familiar insect to anyone who has a mind to shake the branches of oak trees into a net or up-turned brolly. This relatively large leaf hopper (6.5mm to 8.3mm. long) from the family Cicidellidae is wide-spread across Worcestershire and likely to turn up on oak trees almost anywhere in the county (01). However its rarer cousin Iassus scutellaris has become established in the south of the county living in elm hedges.
On 24th July 2014 while surveying hedgerows at Vale Landscape Heritage Trust’s reserve Littleton Meadows I found a small Iassus bug and photographed (02) it because “it didn’t look right”. Its wings were more translucent than any I. lanio that I had seen and it was oddly marked. I checked the British Bugs WebSite and found that it strongly resembled I. scutellaris a Nationally Notable A species found on elm. A check of the distribution on NBN Gateway suggested it to be a species restricted to south east England and East Anglia.
A check on the Auchenorrhyncha Recording Scheme WebSite revealed that the species is now more widely distributed and gives the following account:
“Discovered in Surrey in 1978, this species [Iassus scutlellaris] is now found widely across southern and central England despite its classification as Nationally Notable A. Associated with English Elm Ulmus procera and able to persist on low re-growth following dieback due to Dutch elm disease, it is similar in appearance to the common oak-feeding I. lanio but the colour of the forewings is generally a much brighter lime-green.”
I forwarded my record and photograph to Alan Stewart the Auchenorrhynca Recording Scheme Organiser who gave this cautionary reply:
“…..The distinction between Iassus scutellaris and Iassus lanio is a rather subtle one. …… Externally, it is mainly a question of the pointedness of the vertex (top of head), which I can’t really see on the photo. The fact that it was on elm is highly suggestive of I. scutellaris, but not completely reliable unfortunately as I. lanio can sometimes be found on this tree, although its main host is oak. I have a record of scutellaris from SP24 so not that far from where you found yours. I think this is one of those species for which one would need to examine a specimen to be absolutely sure; getting the right angle on a photo to see the necessary features is extremely difficult …… I would be reluctant to add the records to the recording scheme database without someone inspecting a specimen”.
Unfortunately I had not kept a specimen and a return visit to the location failed to turn up any further examples. However I had been given permission to visit the Worcestershire Wildlife Trust reserve of Hill Court Farm and I took the opportunity to beat some of the hedgerows for invertebrates. I collected several individuals of what I believed to be I scutellaris. I photographed (03) some of the bugs to show the variation and kept one as a specimen. This I pinned alongside a specimen of I.lanio for comparison (04). A clear difference can be seen in the vertex of the two species; I. scutellaris appears to have a longer ‘nose’ because of the shape of the vertex. It is also a smaller insect and in life is a paler more translucent green. The brown markings are variable but distinct from I. lanio. Iassus scutellaris has undoubtedly become established in the county recently but has remained undetected, possibly because of its similarity to its common cousin.
References:
British Bugs WebSite: www.britishbugs.org.uk
Auchenorrhyncha Recording Scheme WebSite: http://www.ledra.co.uk/
Wilson, Michael R. 1981, Identification of European Iassus species (Homoptera: Cicidellidae) with one new species to Britain. Systematic Entomology 6:115-118
Biedermann, R.& Niedringhaus, R. 2009. The Plant- and Leafhoppers of Germany. Identification Keys for all species.

01. Iassus lanio Lower Smite Farm 20 July 2014. Gary Farmer

02. Iassus scutellaris 24 July 2014 Littleton Meadows. Gary Farmer

03. Iassus scutellaris 20 August 2014 Hill Court. Gary Farmer

04. Iassus scutellaris (right) & Iassus lanio (left) pinned specimens. Gary Farmer
Worcestershire Record | 37 (November 2014) page: 20-21 | Worcestershire Biological Records Centre & Worcestershire Recorders
Farmer, Gary - The large pill woodlouse Armadillidium depressum Brandt, 1833 found in Worcestershire
Worcestershire Record | 37 (November 2014) page: 25-26 | Worcestershire Biological Records Centre & Worcestershire Recorders
The large pill woodlouse Armadillidium depressum Brandt, 1833 found in Worcestershire
Gary Farmer
On 8th July 2014 I noticed a Pill Woodlouse on the wall of the Volunteer Centre in the middle of Evesham. This particular individual was slate-grey and very large and on closer examination its body plates (pleonites) splayed out at the edges. When I tried to capture it, rather than rolling into a ball typical of the pill woodlice, it clamped down with its splayed plates covering its legs. This curious behaviour and large size was reminiscent of a species that I am familiar with from the quarries on Portland, Dorset, Armadillidium depressum, so I decided to take the woodlouse to check it later. I referenced A Key to the Woodlice of Britain & Ireland (Hopkin, 1991) which gives the description for A. depressum “….and often rest in a clamped position in which they remain even when disturbed…….pleonites appear splayed out like a skirt….”. Details shown of the uropods and telson from the rear confirmed that I had found Armadillidium depressum. The images 01, 02, 03, & 03 compare the common Pill Woodlouse Armadillidium vulgare with Armadillidium depressum.
This is a strongly synanthropic species and appears to be going through a range expansion. The Atlas of Woodlice and Waterlice in Britain & Ireland (Gregory, 2009) gives the distribution as having a “distinct south-west bias” and a large population is known to exist around Bristol (B. Westwood pers. comm.). So this is certainly a species to look for around the county’s towns and old churches.
Interestingly Worcestershire Biogical Records Centre holds two other records:
Bredon Hill, SO9438, 1985-1986, Paul Whitehead.
Newland SO791482, 29.06.14, John Dodgson.
References
Hopkin, S.P. 1991. A Key to the Woodlice of Britain and Ireland. Field Studies vol 7 No. 4 Field Studies Council, Shropshire.
Gregory, S. 2009. Woodlice and Waterlice (Isopoda: Oniscidea and Asellota) in Britain and Ireland. NERC Centre for Ecology and Hydrology, Oxfordshire.

01. Armadillidium vulgare. Gary Farmer

02. Armadillidium depressum. Gary Farmer

03. Armadillidium vulgare uropods and telson. Gary Farmer

04. Armadillidium depressum uropods and telson. Gary Farmer
Worcestershire Record | 37 (November 2014) page: 25-26 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 26 | Worcestershire Biological Records Centre & Worcestershire Recorders
Two synanthropic Isopods overlooked in Worcestershire
Gary Farmer
Two distinctive woodlice which appear to be strongly synanthropic in Worcestershire are worth looking for in gardens.
Androniscus dentiger Verhoeff, 1908. (01)This is perhaps our most distinctive species being brightly coloured orange or salmon-pink. It is fairly small measuring up to 6mm, fast moving and strongly heliophobic; disappearing quickly when disturbed. This species needs damp conditions and I have only ever found it in deep gravel, under regularly watered plant pots and deep down in the footings of buildings. I am sure it is overlooked in the county because of its choice of habitat so it is worth looking in appropriate places in any garden in limestone areas or close to buildings where lime has leached from mortar or concrete, although I appreciate that not everyone will want to dig out footings just to look for woodlice.
Porcellionides pruinosus (Brandt, 1833). (02). Another distinctive species with long antennae and a fairly narrow body. Porcellionides pruinosus is another fast moving woodlouse with long pale legs which can contrast strongly with the brown/purple body. This is a medium sized species growing up to 12mm but its most distinctive feature is that it often has a bluish powdery bloom. I have only ever seen P. pruinosus in compost bins. Steve Gregory (2009) notes that the species is readily moved around in farmyard manure. So another one to check for in your own gardens, farms and stables.
Reference
Gregory, S. 2009. Woodlice and Waterlice (Isopoda: Oniscidea and Asellota) in Britain and Ireland. NERC Centre for Ecology and Hydrology, Oxfordshire.

01. Androniscus dentiger. Gary Farmer

02. Porcellionides pruinosus. Gary Farmer
Worcestershire Record | 37 (November 2014) page: 26 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 8-9 | Worcestershire Biological Records Centre & Worcestershire Recorders
Wasps in a box: Dolichovespula sylvestris using a bird nest box
Gary Farmer
I was asked to advise on the action to take regarding a wasp nest in a bird box on a house wall in Bishampton (01, 02 & 03). I was curious as I couldn’t recall seeing wasp nests in bird boxes other than Hornets Vespa crabro. When I first saw the nest on 20th June 2014 the wasps were already very active and were extending their remarkable ‘paper’ structure over the outside of the bird box. I was able to take a few reference pictures and could see that they were in fact Tree Wasps Dolichovespula sylvestris. I had not seen the nest of this species before and was unable to find references in ‘the books’ so I did an internet search on “tree wasp nest” to see if this was usual behaviour for them. Unfortunately the internet is more interested in exterminating our black and yellow hymenopteran neighbours than it is on sharing real information. Site after site reported that tree wasps are aggressive and gave details of how to deal with a nest. Sadly it seems that any wasp that nests in trees or bushes is classed as a tree wasp and seen as something to be feared and destroyed. In my experience D. sylvestris is by far the least aggressive of the social wasps and I was happily able to persuade the home owner to let the wasps live. A little later in the summer I was given an update that the wasps were busy eating fence posts and patio furniture and by 17th July the nest was finished and the colony had dispersed.
I have since been able to find reference to various wasp nests in Bees and Wasps by Jiri Zahradnik in which he states that Tree Wasp nests are “…built most often under eaves, in bird boxes, in tree tops or partly underground”. Interestingly in the description of the wasp he notes “It does not bother man and does not enter his homes.” Thank goodness for books.
Reference:
Zahradnik, J. 1998. Bees and Wasps (English edition), Blitz Editions, Leicester.

01. Tree Wasp Dolichovespula sylvestris nest in bird box. Gary Farmer

02. Tree Wasp Dolichovespula sylvestris nest in bird box

03. Tree Wasp Dolichovespula sylvestris nest in bird box. Gary Farmer.
Worcestershire Record | 37 (November 2014) page: 8-9 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 32-33 | Worcestershire Biological Records Centre & Worcestershire Recorders
Albino Starling at Hampton, Evesham, 2014
Harry Green
On 18 June 2014 Betty and Toby Keane saw a ‘white bird’ in their garden in Hampton Evesham (01). It was a nearly perfect albino Starling in brand new barely worn plumage of a juvenile not long fledged and an albino but for the dark eyes and legs. A picture and note appeared in the Cotswold and Vale Magazine (August 2014 issue 179) and shortly afterwards Anne Jordan also from Hampton reported presumably the same bird with four normally coloured siblings being fed by adults on her lawn (02, 03, 04). The albino was ‘the boss ’demanding food from parents before its siblings. Later in summer both albino and the other juveniles left the gardens and presumably joined other starlings in the usual late summer flocks.
In mid-July Donna Saxton reported a very similar bird perched on a TV aerial in Charlton, about two miles from Hampton. (05). Perhaps the same bird although the quality of the picture was too poor for close comparison (05).
Albino Starlings are not often reported.

01. Albino juvenile starling Hampton, Evesham June 2014. Toby Keane

02. Albino starling fed by parent Hampton, Evesham June 2014. Anne Jordan

03. Albino starling juvenile Hampton, Evesham June 2014. Anne Jordan.
 __
__
04. Albino starling juvenile with normal siblings Hampton, Evesham June 2014. Anne Jordan
05. Albino starling at Charlton July 2014. Donna Saxton
Worcestershire Record | 37 (November 2014) page: 32-33 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 67-68 | Worcestershire Biological Records Centre & Worcestershire Recorders
Field recording days 2014
Harry Green
Three special field recording days were arranged for the summer of 2014 organised by Worcestershire Recorders and supported by Worcestershire Biological Records Centre, the latter managing the records on their database.
Black House Wood, Suckley, 7th June 2014, central grid reference SO733521.
An ancient woodland site lying on a Silurian limestone ridge. The wood is well-known for a rich flora but records for other groups relatively few in number. An important site for both Small-leaved and Large-leaved lime. Partly planted with conifers in the past. The wood is being purchased by Worcestershire Wildlife Trust as a reserve and the Trust kindly gave permission to visit.
Pound Green Common, Button Oak, Wyre Forest, 5th July 2014, central grid reference SO754789
Pound Green Common is an important heathland site in the process of rehabilitation and recently acquired by Worcestershire Wildlife Trust. Many thanks for Worcestershire Wildlife Trust for permission to visit.
Grafton Wood, Grafton Flyford, 2nd August 2014, central grid reference SO972560. A visit to a large area cleared of conifers three years previously.
Grafton Wood is an ancient woodland site managed and jointly owned by Worcestershire Wildlife Trust and Butterfly Conservation for many years. Three years previously two large blocks of conifers were clear-felled and this visit was aimed at those areas. Many thanks to both owners for giving permission to visit.
The striking feature of all three recording days was rain. It was raining when we arrived at Black House Wood and only stopped for a short period in the afternoon (pictures 01, 02, 03). Despite this over 300 records were sent to WBRC. The rain had stopped when we arrived at Pound Green Common and the day was cloudy with occasional shafts of sunshine. However the vegetation was wet making invertebrate sampling difficult (04, 05, 06). We arrived at Grafton Wood after a night of torrential rain which slowly stopped as we crossed the fields to the wood. Again the vegetation was extremely wet making invertebrate recording difficult (07, 08). By far the wettest recording year we have ever experienced.
The aim of the field recording days is to obtain as many records of as many groups of plants and animals as possible to give a ‘snapshot’ for the day. Whether we succeed in this aim or not depends on who attends on the day and the weather conditions. 25-27 people booked for each recording day 2014 but the weather took its toll and attendance was 14, 21 and 9 people. Nevertheless a good range of expertise was present with over 300 records for the first two days but around half that for the third day.

01. Mick Blythe and Dave Scott Black House wood. Nicki Farmer.

02. Lunch Black House wood. Nicki Farmer

03. Invertebrate sampling with brolly. Nicki Farmer

04. Recorders at Pound Green Common. Nicki Farmer.

05. Recording day 05July2014 Pound Green Common Mike Averill & Dave Scott & Brett Westwood. Harry Green

06. Recording day 05July2014 Pound Green Common, Rosemary Winnall & Brett Westwood. Harry Green

07. Grafton Wood recording day in wet clearfell. Harry Green.

08. Grafton Wood recording day lunch in iron storage container. Harry Green.
Worcestershire Record | 37 (November 2014) page: 67-68 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 24-25 | Worcestershire Biological Records Centre & Worcestershire Recorders
More records of Western Conifer Seed Bug Leptoglossus occidentalis in Worcestershire
Harry Green
Since John Holder’s record on 4th December 2011 (Holder 2011) there have been occasional further records including the following two.
Jean Young wrote “We came across this lovely specimen of a Western Conifer Seed bug in our bedroom curtains at Besford Court Estate on the morning of 23 October 2013. The window was open overnight so presumably it had come in looking for a cosy place to overwinter. We have several conifers on the estate. We have not seen any others since our ‘indoors’ sighting”. Besford Court Estate Grid Reference SO 9148 4533. (01, 02, 03)
And John Cox wrote “I attach a photo of, I think, a Western Conifer Seed Bug found on our landing carpet on the evening of 30 December 2014 Sadly it was lacking one hind leg”. Kidderminster SO 845756. (04)
It is worth looking out for this large bug. Its origin is explained in the Editor’s Note added to John Holder’s original note and is copied below.
Editor’s note
The British Bugs website http://www.britishbugs.org.uk/heteroptera/Coreidae/leptoglossus_occidentalis.html states “Native to the USA and introduced into Europe in 1999, it has since spread rapidly and during 2008-2010 influxes of immigrants were reported from the coast of southern England, with a wide scatter of records inland. The bug feeds on pines and is likely to become established here; nymphs have been found at several locations. It is attracted to light and may enter buildings in search of hibernation sites in the autumn”. http://www.britishbugs.org.uk/heteroptera/Coreidae/Leptoglossus_occidentalis.pdf a fact sheet from Forest Research gives more information.
Reference
Holder, J. 2011. Western Conifer Seed Bug Leptoglossus occidentalis in Droitwich. Worcestershire Record 31:21.

01. Western Conifer Seed Bug 23 October 2013. Jean Young.

02. Western Conifer Seed Bug 23 October 2013. Jean Young.

03. Western Conifer Seed Bug 23 October 2013. Jean Young.

04. Western Conifer seed bug Kidderminster 30 December 2014. John Cox.
Worcestershire Record | 37 (November 2014) page: 24-25 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 28-29 | Worcestershire Biological Records Centre & Worcestershire Recorders
Bryophytes of Hollybed Farm, Malvern
Ann Hill
Worcestershire Bryophyte Group and the Border Bryologists held a joint bryophyte recording day on Sunday 16th March 2014 at Hollybed Farm, Malvern. Hollybed Farm is a newly established Worcestershire Wildlife Trust grassland reserve of 16ha with a mixture of limestone, neutral and wet grassland, a small valley with calcareous banks, damp woodland, scrub and open grassy areas, small fields of rough calcareous grassland, agriculturally improved fields bordered by hedgerows and the remnants of an old orchard. Fifty bryophyte species were recorded during the day.
The greatest bryophyte interest was found in the damp woodland along the stream valley and it was here that the most interesting bryophyte record of the day was made. The liverwort Cololejeunea minutissima Minute Pouncewort (01) was found by Rita Holmes growing on ageing coppiced hazel at the south-eastern end of the valley. The liverwort is minute (plants to 4(8) mm long with leafy shoots 250-500ums wide) and there were only two previous records for the county: in 2003 at Wissett’s Wood and in 2004 at Broad Down, Malvern.
Another interesting record along the stream valley was of Palustriella falcataClaw-leaved Hook-moss, a pleurocarpous moss indicative of base-rich wet habitats, wet calcareous rocks and seepages. There are very few records of Palustriella falcata in VC37. In the same habitat Oxyrrhynchium schleicheriTwist-tip Feather-moss was also recorded: this is a pleurocarpous moss of well-drained soil on banks by rivers, streams, tracks and lanes, and has been infrequently recorded in the county.
General bryophyte cover was patchy along the stream valley and varied in response to small-scale changes in topography and moisture. In places there was extensive cover of Brachythecium rutabulum Rough-stalked Feather-moss (and in wetter places Brachythecium rivulare River Feather-moss) frequently growing with Kindbergia praelonga Common Feather-moss over the bare soil.
Epiphytic mosses growing on tree trunks and inclined branches included frequent Orthotrichum affine Wood Bristle-moss and more rarely other species of Orthotrichum such as O. diaphanum White-tipped Bristle-moss, O. lyellii Lyell’s Bristle-moss (02) and O. pulchellum Elegant Bristle-moss. Ulota phyllantha Frizzled Pincushion (03), Zygodon viridissimus Green Yoke-moss and Cryphaea heteromalla Lateral Cryphaea were also noted as were the liverworts Frullania dilatata Dilated Scalewort and locally abundant Metzgeria furcata Forked Veilwort.
Bryophytes were scarce in the grassland habitat. A few arable bryophytes such as Barbula unguiculata Bird’s-claw Beard-moss, Bryum dichotomumBicoloured Bryum and B. subapiculatum Lesser Potato Bryum were recorded around the field margins. VC37 only has a very few records of the acrocarpous moss B. subapiculatum possibly because certain identification requires you to free the rhizoids of soil in order to examine the tiny tubers (like minute potatoes) attached to the rhizoids. Bryophytes of arable fields are small and inconspicuous and tricky to identify until you “get your eye in”. Funaria hygrometrica Common Cord-moss, a moss of disturbed and cultivated ground and often found on sites of fires was recorded on bare soil in the corner of a field. Of interest were the large dark green patches of the leafy liverwort Porella platyphylla Wall Scalewort that covered the base of an old ash tree in a hedgerow of one of the fields.
The old orchard was found to be of little interest for bryophytes: possibly because of past management practices or because the fruit trees were pear rather than apple (apple trees have a base-rich bark). However, Hypnum cupressiforme Cypress-leaved Plait Moss was locally abundant on the bark of some of the old trees and generated an interesting discussion about the complexities and identification of species of the Hypnum cupressiformecomplex.
Overall, the farm had a reasonable diversity of bryophytes and we had a very enjoyable and interesting recording day and enjoyed beautiful warm weather.
Additionally, in the morning we saw an interesting and unusual cloud formation over Starling Bank. The cloud was a Lenticular cloud Altocumulus lenticularis – a type of lens-shaped cloud that sometimes forms over mountains and high ground (04 photo taken by Bill Dykes).
We also recorded an adult slow-worm basking on a south-facing bank overlooking the stream valley.
The group was led by Mark Lawley and Ann Hill and the bryologists were Barbara Marshall, Bill Dykes, Des Marshall, Gary Powell, Gillian Driver, John Marshall, Mary Singleton, Pam Parkes, Rachel Kempson, Ralph Martin, Richard Finch, Rita Holmes, Roger Parkes, Tessa Carrick, Wendy Clarke and Xiaoqing Li.
Worcestershire Bryophyte Group is a small informal group that goes out to Worcestershire sites to record and learn about bryophytes. Our broad aim is to assist everyone, especially those who are new to mosses and liverworts, to become more experienced and confident at identifying bryophytes. Beginners are always very welcome, the only equipment needed is a hand lens (x10 or x20) and some paper packets for collecting specimens. Below are dates for our next planned field excursions. If you are interested in joining us on either of the field trips please contact me at ann@gaehill.f9.co.uk and I will let you know full details.
Table 1. List of Bryophyte species recorded at Hollybed Farm, Malvern 16thMarch 2014.
| Latin Name | English Name |
| Mosses | |
| Amblystegium serpens var. serpens | Creeping Feather-moss |
| Barbula unguiculata | Bird’s-claw Beard-moss |
| Brachythecium rivulare | River Feather-moss |
| Brachythecium rutabulum | Rough-stalked Feather-moss |
| Bryum capillare | Capillary Thread-moss |
| Atrichum undulatum var. undulatum | Common Smoothcap |
| Platyhypnidium riparioides | Long-beaked Water Feather-moss |
| Bryum dichotomum | Bicoloured Bryum |
| Bryum subapiculatum | Lesser Potato Bryum |
| Cryphaea heteromalla | Lateral Cryphaea |
| Dicranoweisia cirrata | Common Pincushion |
| Didymodon insulanus | Cylindric Beard-moss |
| Didymodon rigidulus | Rigid Beard-moss |
| Fissidens bryoides var. bryoides | Lesser Pocket-moss |
| Fissidens taxifolius var. taxifolius | Common Pocket-moss |
| Funaria hygrometrica | Common Cord-moss |
| Grimmia pulvinata | Grey-cushioned Grimmia |
| Hygroamblystegium tenax | Fountain Feather-moss |
| Hypnum cupressiforme var. cupressiforme | Cypress-leaved Plait moss |
| Hypnum cupressiforme var. resupinatum | Supine Plait-moss |
| Isothecium myosuroides var. myosuroides | Slender Mouse-tail Moss |
| Kindbergia praelonga | Common Feather-moss |
| Leptodictyum riparium | Kneiff’s Feather-moss |
| Mnium hornum | Swan’s-neck Thyme-moss |
| Neckera complanata | Flat Neckera |
| Orthodontium lineare | Cape Thread-moss |
| Orthotrichum affine | Wood Bristle-moss |
| Orthotrichum diaphanum | White-tipped Bristle-moss |
| Orthotrichum lyellii | Lyell’s Bristle-moss |
| Orthotrichum pulchellum | Elegant Bristle-moss |
| Oxyrrhynchium hians | Swartz’s Feather-moss |
| Oxyrrhynchium pumilum | Dwarf Feather-moss |
| Oxyrrhynchium schleicheri | Twist-tip Feather-moss |
| Palustriella falcata | Claw-leaved Hook-moss |
| Plagiomnium undulatum | Hart’s-tongue Thyme-moss |
| Rhynchostegium confertum | Clustered Feather-moss |
| Syntrichia montana | Intermediate Screw-moss |
| Thamnobryum alopecurum | Fox-tail Feather-moss |
| Tortula muralis | Wall Screw-moss |
| Ulota species | |
| Ulota phyllantha | Frizzled Pincushion |
| Zygodon viridissimus var. viridissimus | Green Yoke-moss |
| Liverworts | |
| Cololejeunea minutissima | Minute Pouncewort |
| Conocephalum conicum | Great Scented Liverwort |
| Frullania dilatata | Dilated Scalewort |
| Lophocolea bidentata | Bifid Crestwort |
| Lophocolea heterophylla | Variable-leaved Crestwort |
| Metzgeria furcata | Forked Veilwort |
| Pellia epiphylla | Overleaf Pellia |
| Porella platyphylla | Wall Scalewort |
| 01. Cololejeunea minutissima Minute Pouncewort. Ann Hill |
| 02. Orthotrichum Lyellii Lyell’s Bristle-moss. Ann Hill |
| 03. Ulota phyllantha Frizzled Pincushion. Ann Hill |
| 04. Lenticular cloud Altocumulus lenticularis. Bill Dykes |
Worcestershire Record | 37 (November 2014) page: 28-29 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 22 | Worcestershire Biological Records Centre & Worcestershire Recorders
An interesting cellar with bats, birds and butterflies
Garth Lowe
My long term study of swallows in Alfrick on the edge of west Worcestershire, takes me into odd places including a large cellar where Swallows have been nesting for many years. In the middle of August 2014 while checking a nest that had already produced chicks, the owner told me about other surprising occupants.
In the gloom at the back section my torch lit up a Lesser Horseshoe Bat hanging like the proverbial plum! (01). It failed to wake up for the few minutes that we were there, and was obviously well into “shut down mode”. Small movements from it indicated it was definitely alive: but why had it gone into hibernation mode in a summer month? The weather had cooled down from previous weeks but not so critical that it would send a bat to sleep. By the pile of droppings beneath it, this must be a favourite place for it to hang out safely.
Right at the back of the cellar more sleeping creatures were found hanging on the ceiling. There must have been half a dozen each of Small Tortoiseshell and Peacock butterflies looking like they were heading for a very long wait till spring. It may be that having located the cellar they reawaken on good autumn days and go outside to top up on available nectar sources. One hopes the sleeping bat does not wake up to find them for a snack!
Sleeping with the butterflies were also a few of the only moth we have that hibernates in the adult form, the Herald; they too have a long wait until spring arrives. This is a fairly common moth with distinctive wing shape and wing markings and can also be the first moth to be seen every year and possibly also the last (02).
One month later another visit was made to check the Swallows had all flown, only to find probably the same bat still there. This time it was fully awake and found hanging in another spot and then fluttered around in the gloom. It appears that when it goes torpid it must hang in exactly the same place as there was a distinct black patch of dropping on the ground beneath it.
On this visit there were in fact butterfly wings lying around, one being from a Red Admiral, so it does look as though the bat snacks on them, probably when they fly in during the day and the bat is awake and picks them up on its echo location system! A better inspection is needed when the bat is not in residence to comply with the restrictions on people entering a known bat roost. Other butterflies and Heralds were still clinging to the ceiling, so they may have been the lucky ones that flew in or out while the bat was fully asleep. One Peacock was aroused by my movement and maybe torchlight and it did the noisy flicking of wings and flashing the beautiful eye spots that they do to deter predators while they are torpid.
A final check at the end of October found a single Tortoiseshell and 16 Herald moths: there were probably more tucked away in the timbers, but the fact there were also two Lesser Horseshoes fast asleep and not wanting to disturb them time to explore further was limited.
Other temporary inhabitants of the cellar back in the spring had been a family of Wrens. The lazy or smart male, whichever way you look at it, had used an old swallow nest attached to a beam, and only had to put a roof on it to try and attract a female. Male Wrens are of course quite unusual in as the male does the building of more than one nest and takes the lady of his choice on a round of inspections! This time he was successful in persuading her that this was a secure site. Interestingly both the Wren and a pair of Swallows nested very close together, with only a main supporting beam between them!
In the past both Robins and Blackbirds have also found the cellar a safe place to rear a family, so that makes a grand total of seven species using this damp gloomy underground space with an approximately five foot wide door at the bottom of some steps.

01. Lesser Horseshoe Bat hanging like the proverbial plum. Garth Lowe

02 Peacock butterfly and Herald moths. Garth Lowe.
Worcestershire Record | 37 (November 2014) page: 22 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 47 | Worcestershire Biological Records Centre & Worcestershire Recorders
New record in Worcestershire – Chamaesyce serpens
Bert Reid
Chamaesyce serpens, previously known as Euphorbia serpens, is a species in the Spurge family Euphorbiaceae known by the common name matted sandmat. Originally native to South America,
It is now found across much of the world as an introduced weed. It forms a mat of prostrate stems which root at nodes where the stem comes in contact with the ground. The detailed structure of Spurge flowers are always hard to understand, even in our common species, and to me they are impossible with tiny unfamiliar aliens.
On the 29th August 2014 I was recording plants along Longdon Hill near Wickhamford, Evesham. I finished my recording and was walking back to where my car was parked when I passed by the garden center Vale Exotics. In a fit of curiosity I popped in looking at the Tree Ferns and other interesting plants and happened to notice a big patch of weeds stretching on to the path around the display. I didn’t recognize what it was, so I spoke to the staff there who said that they certainly didn’t plant it there and had regularly tried to get rid of it. I collected a bit to take home, but failed to identify it. My eyesight does not allow me to use a camera so I asked Harry Green to get a close-up photo of the plants I collected. I could then see the details on my computer, but after ruling out all the plants I suspected, I decided to send Harry’s photos (01, 02) to Quentin Groom, an expert at the Botanical Society of Britain & Ireland.
Next morning I got a reply determining the identity. The reply made me feel a bit better for my failure, because I had never even heard of the species or even the current genus. The only earlier records in Britain I have found were rare (less than 5 sites) sporadic plants from imported bird seed and / or wool waste on tips.

01. Vale Exotics with mat of Chamaesyce serpens. Harry Green

02. Highly magnified section of Chamaesyce serpens. Harry Green
Worcestershire Record | 37 (November 2014) page: 47 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 48-50 | Worcestershire Biological Records Centre & Worcestershire Recorders
The Flora of Worcestershire – Notes and Additions
Bert Reid
I cannot write an unbiased critique of Roger Maskew’s book, The Flora of Worcestershire 2014, because I have been strongly involved in the Worcestershire Flora Project from the inaugural meeting in 1987, through the creation of a charity in 1994, up to my withdrawal from the management of the project and charity in 2005. In my letter to the Committee, I wrote that “Both Roger and I are strong-willed people with firm ideas, and we disagree about many aspects of the project. The resolution of such differences can only occur if there is a wider body to which the issues and arguments can be referred. If this is to be a collegiate project, I think that it needs collegiate governance.” I also wrote “Any removal or addition or maps would require a full rewrite of the species concerned and I would not be happy for this to be carried out by anyone else. Individual authors must have the final say over accounts published in their name”.
The author will thus not be surprised that I have some reservations about the book, since we have discussed most of these over a number of years. The book is too bulky and the quality of the binding is far from ideal. Although I agree with Roger that the book should be made available in printed form, I hope that the book will be made available on the internet (as a pdf) as soon as is practical. The book would be easier to use if an Ordnance Survey Map of the County was included. Not everyone knows where the places named in the accounts are. I do not think that the brief mention of NVC classifications is adequate. I know that the author does not appreciate them, but many international and professional readers will, and I don’t see the problem with a brief entry like my soil summary in the first chapter. We had long arguments over maps, and I eventually agreed with the compromise, but am disappointed with the quality of the maps – I can hardly see the hectad lines, but that may be my declining eyesight! I would have preferred the inclusion of Charophytes, which are covered by BSBI and are well known in the county: our records include nationally important species. The Extinctions and Changes chapter was originally written by me but the changes and rewrites by Roger made the account so confused and difficult to follow that I refused to be published as a joint author.
The proof reading is generally excellent, but it was unfortunate that the rediscovery of Pyrola minor was attributed to P.L. Reake rather than P.L. Reade. I think that brief curriculum vitae of the major contributors to the flora (including the author) should be added. We know how difficult it is to find any details of historic people from herbaria specimens and the like and we ought to assist future readers to find a little bit out about us.
The book as a whole is curiously unbalanced and old fashioned. With little or no coverage of plant communities, landscape history, bryophytes, archaeophytes, IUCN status etc the book could have been written in the 1980s. The 2013 Flora of Birmingham and the Black Country is very different in coverage but is a good example of how a lot of data (and photographs) can be presented in an attractive and readable way.
Post-flora Records
There have been several species found since the publication of the flora. I am ignoring the Stoneworts, as these need a separate article at a later date. The taxa below are in alphabetical order of Latin name, rather than the scientific sequence used in the book. Some of the taxa included here are not recent records but were excluded from the book as being “hortal species and cultivars in gardens, the cultivated parts of churchyards, cemeteries, allotments, urban parks and planting schemes…” but these rules are not followed consistently. Roger Maskew’s record for Malus transitoria clearly planted on a roadside opposite the Commandery Museum in Worcester is included (with a photograph!) yet records such as the first species below are not included. Here I include such plants. All the records are introduced unless otherwise noted.
Acer platanoides Schwedleri group. Purple Norway-maple.
The Purple Norway-maple is a clear introduction, but Keith Barnett noted numerous seedlings from a large planted tree in the communal garden at Lansdowne Crescent, Malvern, in April 2003, grid SO7846. With clear evidence of reseeding and probable naturalisation it is worth including. One site noted.
Amaranthus hypochondriacus L. Prince’s-feather
Princes-feather is a domestic species, developed in cultivation as an attractive garden plant related to the more common A. caudatus, Love-lies-bleeding. The only record away from a garden was by Keith Barnett, who found a single plant in flower on the pavement of Abbey Road, Malvern in August 2010, SO7745.
Anthemis austriaca Jacq. Austrian Chamomile
This rare but increasing alien was first found by A.W. Reid in September 2009. It was noted with a very unusual mix of plants of clearly differing origins on an earth bank between the old and new roads on the B4624 Evesham Road, SP028459. There were two non-flowering plants, confirmed by C.P. Poland and E.J. Clement from a photo of a grown-on flowering plant. The plants flowered again in 2010 and my experience allowed me to confirm the next record, this time in 2012 by Keith Barnet on the B4211, Hanley Castle, SO839423. About 100 plants were flowering there on a grass or wildflower seeded verge.
Apium graveolens var. dulce (Miller) DC Celery.
This is another record from the earth bank between the old and new roads on the B4624 Evesham Road, this time in 2010. The grid reference and recorder were as my Anthemis austriaca but this plant clearly originated from market garden soil.
Betula utilis var. jacquemontii Spach. Jacquemont’s Birch.
This unusual tree was obviously planted in the Pershore Bridge picnic area (SO952449). It was recorded by A.W. Reid in 2010. It is an interesting and attractive tree with brilliant white bark, and is becoming popular in roadside plantings.
Brassica “Mizuana” Japanese Greens.
This is yet another record by A.W. Reid from the earth bank between the old and new roads on the B4624 Evesham Road in 2010 with the very close grid reference of SP027460. This plant is easily recognised as a market garden plant grown for salads but I discussed the find with Tim Rich to see if he could give me an accepted Latin name: in his reply he says he is “inclined towards rapa as the ‘species’ but I really don’t know”.
Calla palustris L. Bog Arum
Bog Arum is described by Stace as “Intrd-natd; grown for ornament, persistent and spreading in marshy ground and shallow ponds”. We only have a single record, when Mr J.J. Bowley noted it as planted in Kings Heath Park pond, SP067816, date February 1990.
Capsicum annuum L. Sweet (Chilli) Pepper
This is a very strange record by A.W. Reid from an arable field north of Bredons Norton, SO932396, date July 2013. I was walking along the track looking for arable weeds when I noticed a patch of disturbed ground about 10m into a field of rape. I struggled through the rape to investigate and was astonished to see about 20 Chill Peppers growing in fruit. I then walked all around the field but found no more Chillies, though there were a few Phacelia, Buckwheat and White Mustard along the more distant edges. The source of the plants is uncertain, but I can only guess that a nearby neighbour had cleared out a domestic greenhouse and thrown the rubbish into the rape field.
Chamaesyce serpens (Knuth) Knuth. Matted Sand-mat
This is the most recent, difficult, confusing and ultimately satisfying find, by A.W. Reid on the 15th September 2014. I was plant hunting along Longdon Hill, Wickhamford, where among other things I found Blue Fleabane and Waterer’s Cotoneaster. I was walking back to the car when I noticed the Vale Exotics garden centre (SP062416) and out of curiosity I popped in to have a look. I saw the Tree Ferns and the like and was just leaving when I spotted an odd weed that I didn’t recognise. It was a sprawling mat, quite unlike anything I’d seen before, so I asked the staff if they had planted it or knew what it was. They said they hadn’t and found it nuisance weed, hard to get rid of and they were happy for me to take a bit. (see note elsewhere in this number of Worcestershire Record.
Crocus chrysanthus (Herb.) Herb. Golden Crocus
The Golden Crocus has often been recorded in error for Crocus x stellaris, the Yellow Crocus. Earlier records have all been corrected or marked as requiring confirmation. However, in March 2013 John Day found the genuine plant when checking churchyards. At St Peter’s Church, Droitwich, SO902625, he found a small colony of about 20 flowering plants in grassland east of the church, established and spreading, plus a separate single flowering plant west of the church. He also visited Abberley Church, SO751679, where he noted a small group in grassland southwest of the church.
Diascia vigilis Hilliard & B.L. Burtt. Twinspur
I am surprised that this did not appear in the book. Roger Maskew recorded several plants on a trackside bank at Abberley Lodge, SO749668, in July 2000. The plant was determined by Bill Thompson and had never previously been recorded in the County.
Erica vagans L. Cornish Heath
This is native species, but not in Worcestershire. We have just one acceptable (if not countable) record, by Brett Westwood, who noted it as established in an old cottage site, previously garden, at Town Coppice, Wyre Forest, SO767761, date April 2011. Records like this are worth publishing so that the source of potential future finds is not forgotten.
Eryngium giganteum M. Bieb. Tall Eryngo
This first county record was found by A.W. Reid on Mitton Way, Mitton north of Tewkesbury, SO900336, date July 2013. I found a single plant of this alien garden plant, (probably cultivar ‘White Ghost’) on the pavement edge outside shops. No others were seen anywhere in any garden nearby.
Eryngium planum L. Blue Eryngo
This first county record was found by A.W. Reid on Holloway, Pershore, SO937461, date August 2012. I found a single large plant just coming into flower on the verge at the base of a street light. I have never seen this anywhere else in Pershore, and there was certainly no sign of it in any of the nearby gardens.
Eucalyptus gunnii Hook. f. Cider Gum
This Eucalypt was introduced by the late Fred Fincher near his cottage in Randan Wood (SO9172) with other plants such as Ivy Broomrape and May Lily. Many of these introductions gradually declined and then disappeared, but some could still be found very recently. It is believed that the Cider Gum was planted in about 1975 but was still present in 2000 or later when John Day, Bert Reid, Roger Maskew and others all saw and identified the plants at various times.
Galanthus woronowii Losinsk. Green Snowdrop
This first County record was found by Bert Reid at St Philip & St James Church, Strensham in February 2012, SO910406. A few clumps were growing in the churchyard with abundant G. nivalis. The identification was confirmed by Dr Aaron Davis at Kew from photographs taken.
Gilia tricolor Benth. Bird’s-eyes
The first county record of this attractive garden escape was found by Bert Reid off Queensmead, Bredon Village, SO933369, in August 2013. A small patch was growing at the edge of a closed garage area where an unofficial track enters a footpath.
Ginkgo biloba. Ginkgo
There are three records of this tree. They were: in 1911, by a field meeting of the Worcestershire Naturalists Club at Overbury Park, SO9537: in 2005 by John Day, Bert Reid and Paul Reade on the embankment of the Birmingham Canal Spon Lock to M5, SP001898: and in 2010 by John Day at St Faiths Church Overbury, SO956374.
Helianthemum x sulphureum Willd. ex Schltdl. Hybrid Rock-rose
This hybrid between the locally scarce Common Rock-rose and the nationally rare White Rock-rose is known as occurring as a native in one or two sites, far distant from Worcestershire. The only County record, by Bert Reid in July 2014, clearly relates to the garden escape of a commercial cultivar. I found a single whitish-flowered plant on the edge of the track from Park View Terrace, Barbourne towards the River Severn, SO841569. The plant was a surprising distance from any property likely to be a source of the escape.
Heuchera x brizoides hort. ex Lemoine Coral-bells hybrids
The flora shows five records of Heuchera sanguinea, Coral-bells. A better understanding of the Genus allowed Keith Barnet to deal more appropriately with this recent record. He found a single plant in flower just outside a garden in Hanley Swan, SO812428, in June 2013. The earlier records should in part (and probably in whole) be attributed to the horticultural hybrid.
Hypericum forrestii (Chitt.) N. Robson. Forrest’s Tutsan
This is another surprise omission from the book. Bill Thompson recorded this plant in September 2002, from Hurcott Road, Greenhill, Kidderminster. He noted two self-sewn at the foot of walls, one on the opposite side of the road from the parent bush and the other some 30m. up the road from it.
Hypericum pseodohenryi N. Robson. Irish Tutsan
The Irish Tutsan was recorded by John Day in July 2003, from a shrub belt by a service road of the A491, SO911819. It was locally abundant from likely planted stock surviving and becoming established.
Laburnum x watereri (Wettst.) Dippel. Hybrid Laburnum
Roger Maskew and Chris Westhall recorded this hybrid in August 2003, in the churchyard of Martley Church, SO756599. Roger Maskew determined the identity but did not include the record in the book.
Liriodendron tulipifera L. Tulip Tree
There two old records of the Tulip Tree. The earlier was in 1886 when the Naturalists Club held a field meeting at Areley Castle (SO7680, Staffordshire) and noted “noble specimens” planted in the arboretum. The more recent was in 1980 when John Day noted the tree in Kyre Park, SO6263.
Matthiola incarna (L.) W.T. Aiton. Hoary Stock
The only record is from Keith Barnett, who noted it at the junction of Tibberton Road and Imperial Road near Barnards Green, SO782456, in September 2010. There was one sturdy plant at the foot of a garden wall, present for at least two years.
Narcissus minor L. Lesser Daffodil
Narcissus minor is easily mistaken with small cultivars of the native Daffodil, but Bert Reid decided to study the problem of identification in 2010. I started near to home where I saw a few small discarded daffodils in a layby on the lane from Allesborough to Ladywood, SO939462, and these proved to be the genuine Narcissus minor. Thus emboldened I went to my recent favourite site, an earth bank between the old and new roads on the B4624 Evesham Road, SP028459 & SO027460. There were three genuine plants there split between the two grid references. It showed that garden plant soil had been included in the bank.
Olearia macrodonta Baker. New Zealand Holly
The single record is in 2005, by John Day, Bert Reid and Paul Reade: several planted in a native shrub belt on the embankment of the Birmingham Canal Spon Lock to M5, SP001898.
Phaseolus vulgaris L. French Bean
This is yet another record by A.W. Reid from the earth bank between the old and new roads on the B4624 Evesham Road, in 2010, with the SP028459. The single plant is clearly a market garden plant.
Polystichum munitum (Kaulf.) C. Presl. Western Sword-fern
The first and only record of the Western Sword-fern was a single plant in a shady alley between houses in Bewdley SO782749. It was recorded by Brett Westwood and the Wyre Forest Study Group in September 2012, and determined by Brett Westwood and John Day.
Selaginella kraussiana (Kunze) A. Braun. Krauss’s Clubmoss
This is the first County record for this alien Clubmoss. John Day found it at Tenbury Church, SO594683, in March 2013. It was dominant over several square of shaded trodden area near the vestry door on the northeast side of church.
Silene coeli-rosa (L.) Godr. Rose-of-heaven.
This first County record was recorded by Bert Reid from Overbury village. In June 2013, I walked up to the start of Pigeon Lane, SO960378. Here I saw a small group of flowering plants on a grass bank by a bench seat. I surmise that these were the remnants of an original planting.
Smyrnium perfoliatum L. Perfoliate Alexanders
There are two records for this plant. The first was in 1996 when Brett Westwood was near the edge of Monk Wood, SO798601, and he saw the plants seeding well and establishing themselves just outside the garden. The other record was in the grounds and gardens of Red House, Eldersfield, SO793300, in June 2012, when the Worcestershire Recorders held a field meeting including John Day, Bert Reid, Ann Fells and Brett Westwood. The plants were established and showing signs of natural spread, likely from imported stock.
Sorbus thibetica (Cardot) Hand.-Mazz. Thibetan Whitebeam
This alien Whitebeam was recorded by Chris Westall in October 2000. He noted two trees growing on the edge of the curtilage of Hillcrest School, Woodgate Valley, SP012833. The plants were identified by Tim Rich. They were not obviously planted but were scarcely “in the wild” and were felled at some time before 2009 to make room for additional tennis courts.
Taraxacum cherwellense A.J. Richards. Cherwell Dandelion
This is the most important Dandelion we have collected in Worcester. In April 2012Bert Reid was looking for Taraxacum in the Upton Snodsbury parish and found an unfamiliar one on a wide grass footpath by arable, SO940538. I thought it was an odd T. glauciniforme, being a small dandelion without pollen, so I sent the specimen to John Richards. He was obviously excited by my find, correcting my guess (I was in the wrong Section) and writing “This is a most important record for one of our rarest endemics. Please return and survey!!” I have returned a couple of times in 2013 and 2014, but each time bad weather or holidays abroad have made a detailed search difficult, and I have not yet succeeded.
Taraxacum inopinatum C.C. Haw. Unexpected Dandelion
In May 2012, John Day collected some Erythrosperms from Cambridge Farm, Birts Street, grid SO782367. As usual he gave the specimens to Bert Reid to decide if they worth worth sending off to John Richards. As soon as I saw the specimens I got excited, because I was fairly sure that they included T. inopinatum, a dandelion I had seen away from the County and had half expected to turn up in Worcestershire. When John Richards received the specimens, he confirmed my determination, giving us a new county record of a native plant.
Taraxacum pruinatum M.P. Christ. Pruinose Dandelion
Worcestershire is particularly good for dandelions in Section Hamata of which we have recorded nearly all the British species. I was therefore very pleased when in March 2011; John Day collected an unusual one from the grass verge of the lane from Grafton Flyford to Himbleton, SO961562. I looked at the specimen and tentatively determined it as the rare T. pruinatum, previous known only from the Chester district. John Richards confirmed my determination, giving us another new County record.
Taraxacum remanentilobum Soest. Falcate-lobed Dandelion
This is another first county record for a Taraxacum species. T. remanentilobum is a Ruderalia described by John Richards as an introduced close relative of T. vastisectum with a few records in four vice-counties. John Day found it on a woodland ride in Haws Hill Wood, SO606654, in April 2011. The specimen was determined by John Richards.
Taraxacum subnaevosum A.J. Richards. Pale-bracted Dandelion
This is an endemic dandelion in section Naevosa, frequent and widespread in some areas of northern England and Scotland. We were therefore surprised when in April 2012 John Day found a colony of an unknown Naevosa by the A4189 Henley Road, Oldberrow, SP124648. The colony stretched over about 100m of grass road verge, most but not all on the south side. John collected four plants, that we tried to identify, but our experience and knowledge of northern Naevosa are rudimentary and we chose the wrong one of our final two. Of course John Richards put us right! I returned in 2013 with John Day but very few were in an identifiable state, with leaves poorly developed and most not in flower.
Taraxacum sundbergii Dahlst. Sundberg’s Dandelion
This is a rare section Ruderalia scattered in four vice counties. We added another vice county when John Day found the plant on the hedgebank of Lincomb Lane, SO825692, in March 2012. John Richards determined the plant.
Tradescantia virginiana L. Spiderwort
Spiderwort is a common introduced garden plant, often persisting when thrown out or spreading out of the garden. We have records from eight tetrads (seven hectads – SO77, 85, 87, 93, 94, 96, 98) between 1989 and 2013. A personal example is from Church Walk, Pershore, where my 1998 record says one large plant at foot of wall pushing up through pavement asphalt: known here for at least 10 years. Since then the pavement has been resurfaced but three plants appeared nearby in a rebuilding works.
Verbascum phoemiceum L. Purple Mullein
A 1995 record for Tank Quarry has been removed as an error, but we still have a Terry Knight June 2012 record of a single plant on a grass verge beside a path between Blacksmiths Close and Farm Lane, South Littleton, SP079462. There is no reason to doubt this record.
Weigela florida (Bunge) A. DC. Weigelia
The only record, in July 2013, was recorded by Keith Barnett from St Wulstans nature reserve, SO781413, as a single relic plant in flower.
Zauschneria californica. Californian Fuschia
This final record (alphabetically) gave me quite a lot of difficulty. This first county record was found by A.W. Reid on Mitton Way, Mitton, north of Tewkesbury, SO900336, date July 2013: the same date and grid reference as my Eryngium giganteum. The first difficulty was whether it was countable. It was on a footway edge of shops by a car park area, and escaped from the garden nearby under the fence. The second difficulty was what was it? The garden contained several patches of various Fuchsias and I thought it must be an odd Fuchsia cultivar but a long look on the internet showed nothing similar. Some flower images looked similar, but the very narrow grey-haired leaves were quite unlike any Fuchsia. I was almost giving up when I discovered from an American flower book I had been given that “Californian Fuchsia” was Zauschneria californica. With the different Genus I found a perfect match to my plant. So I resolved my second difficulty, if not my first.
Worcestershire Record | 37 (November 2014) page: 48-50 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 51-53 | Worcestershire Biological Records Centre & Worcestershire Recorders
The Flora of Worcestershire – Other Important Updates
Bert Reid
There are many important locally rare, interesting or threatened taxa that did not get included in the Flora of Worcestershire (Maskew 2014), even if earlier records did. I have not yet been able to work out all such taxa so this list is just a quick selection of them, in no special sequence. The receipt of a pre-publication copy of ‘The Vascular Plant Red List for England’ from BSBI has added a major hurdle in the race to complete this article. The mention of any site or grid reference here does not mean public access is permitted.
Fumaria purpurea Pugsley. Purple Ramping-fumitory
This fumitory is shown as VU (vulnerable) in the recent Red List for England. Keith Barnett found several in Back Lane behind Waitrose, Great Malvern, SO776463, in May 2014. He sent specimens to Rose Murphy (BSBI expert) who confirmed the determination.
Cephalanthera damasonium (Mill.) Druce. White Helleborine
The most recent record of this VU (vulnerable) species was by Roger Maskew in 2011. He found about 45 flowering plants at Hipton Hill, SP026478.
Cephalanthera longifolia (L.) Fritsch. Narrow-leaved Helleborine
Wyre Forest has long been known as an important site of this VU (vulnerable) species. For many years, Rosemary Winnall has taken on the responsibility for organising the detailed recording and monitoring of the plant for Plantlife. The most recent results I have details of show 14 sites spread over five monads, SO7474, SO7475, SO7476, SO7574 and SO7575.
Anthemis arvensis L. Corn Chamomile
This endangered (EN) archeophyte has three post 2009 records: in Woodgate Valley, SO994830, John Day: in Astwood Road Cemetery, SO854565, John Day: and on Bredon Hill, SO966391, Roger Maskew. Only the Bredon Hill site is likely to be long standing.
Astragalus danicus Retz. Purple Milk-vetch
A. danicus has always been rare in Worcestershire. The 2014 English red list has it as endangered (EN). Bredon Hill is the last surviving site, except for one or two known translocations, so it is vital that it is regularly monitored. The 2013 monitoring by Bert Reid had the usual problem of finding the plant in heavily grazed limestone grassland, but I eventually found a small non-flowering patch at the top of the bank, SO944393.
Botrychium lunaria (L.) Sw. Moonwort
The vulnerable (VN) Moonwort is well covered in Roger Maskew’s Flora but he seems to have missed the 2011 record by Rosemary Winnall, one plant on Bolton Meadow, SO771508.
Campanula patula L. Spreading Bellflower
The English Red List gives this as Critically Endangered (CR). It is one of the most endangered plants in England so I give more information of the 13 recent finds than The Flora of Worcestershire can. Keith Barnett surveyed two sites in 2010 returning to one in 2013. The first was Bowling Green Common, Clevelode, SO829466, where he found at least six in flower by a ditch in probably our most visited site. The other site he surveyed was Dripshill Wood, SO829457, where there was one in flower at the summit of the wood. I was pleased that the plant was still present in the site I rediscovered in 2009. The other surveys in 2013 were all carried out by Charlotte Long and Laura Jones, both working on Campanula patula for the National Botanical Gardens Wales using site information from John Day and Bert Reid. They only found a single plant on Bowling Green Common and failed to find any at Dripshill Wood but did succeed better elsewhere. On the track from Durbridge Farm onto the bank above the river Leadon, SO734299, they found a single plant half way along an exposed patch of bank. At the Worcester Golf and Country Club, Broughton Park, SO828536, they estimated 15 to 20 plants (some inaccessible) and collected 8 small samples for DNA analysis. In the wood below Morton Hall, SP017595, they found eight plants along a woodland path. On the bank of the Worcester to Stourbridge railway between Hagley and Pedmore, SO903812, they estimated a minimum of five plants seen from behind high metal fencing around the cutting staircase. They managed to access one plant to take a DNA sample.
Eleusine Africana. African Yard-grass
Today (29/7/2014) I received an exciting letter from Tom Cope, BSBI grasses referee at Kew, confirming the identification. Terry Knight sent me a plant that he thought was E. indica subsp africana. I thought Terry was probably right, but I am a bit out of my depth with alien grasses so I collected a better specimen and sent it off to Tom Cope after reading in his Handbook that the plant had been promoted to a full species. My contribution to the record is purely agreeing with Terry’s identification and sending off a better specimen. So the full details of the record are: collected by T.D. Knight from the bottom of the west kerb of Barnards Close, Evesham, SP042429, 12th September 2014 confirmed (as T.africana) by Dr T.A. Cope from a specimen prepared by A.W. Reid.
Verbascum chaixii x thapsus ? a hybrid Verbascum
Keith Barnett and Roger Maskew had a bit less luck with this. Earlier this year, Keith found a strange yellow-flowered Verbascum on his driveway. Roger kept a close eye on it and saw it was a hybrid so it was sent to Vic Johnstone (BSBI Verbascum referee). He informed Roger that, having weighed up all the options, his ‘best guess’ is Verbascum chaixii x thapsus, most assuredly a new county record. So no definitive determination made.
Carex echinata Murray. Star Sedge
This is a less surprising Red List near threatened plant (NT). It is worth detailing both our post 2009 records. In May 2011 a visit to Great Bog, New Parks, Wyre Forest SO747762 was attended by J.J. Day, B.Westwood, R.A.Winnall, M.W.Poulton, P.L. Reade, J.Bingham and D.Scott. Star Sedge was just one of their good finds. In July 2011, on Berrow Downs, SO764381, K.Barnett found many in a marshy area, in fruit.
Chamaemelum nobile (L.) All. Chamomile
In 2011there were three records from Castlemorton Common of this vulnerable (VU) plant. All were centred around grid SO774385 on different dates recorded by Peter Garner, Keith Barnett, and Roger Maskew.
Erica cinerea L. Bell Heather
This declining near threatened plant (NT) has few recent records. In 2011 the same group who recorded the Star Sedge found Bell Heather by the disused railway at SO744740. The Wyre Forest Study Group (John Day, Rosemary Winnall et al) visited Willow Bank Meadow, SO746733, and found some more. The most recent record, in July 2014, was from Pound Green Common (SO7578 Staffordshire) when Bert Reid et al found both E.cinerea and E.tetrax.
Erica tetralix L. Cross-leaved Heath
As with Bell Heather, this declining near threatened plant (NT) has few recent records. In 2011 John Day monitored the plant on Hartlebury Common and found it in the Bog SO819707-SO820708 and on damp heath SO818705 (locally frequent). The most recent record, in July 2014, was from Pound Green Common (SO7578 Staffordshire) when Bert Reid et al found both E.cinerea and E.tetrax.
Galium parisiense L. Wall Bedstraw
This vulnerable (VU) plant has two post 2009 records. In 2011 Roger Maskew saw it at Kinsham Gravel Pit, SO938367. In the same year, John Day and Adrian Darby found it on the Kemerton Estate grid SO939364 East Gloucestershire. There were about 10 plants scattered over a few sq. metres in open vegetation on sandy soil.
Genista anglica L. Petty Whin
This vulnerable (VU) plant has two 2012 records. Keith Barnett and Roger Maskew found a few scattered plants in flower on Castlemorton Common, SO786390. John Day recorded it on Monkwood Green.
Genista tinctoria L. Dyer’s Greenweed
There are eight records for this vulnerable (VU) plant, all by Keith Barnett or Bert Reid. I have selected the most recent. In 2012 Keith Barnett recorded it at St Wulstans Nature Reserve, Malvern Wells, SO784412, locally abundant in flower and fruit on grassland. In 2013, Bert Reid and Mike Liley found it abundant in Baynhall Meadow, SO981531.
Helianthemum nummularium (L.) Miller. Common Rock-rose
This near threatened (NT) species has eight recent records but is selected as an important locally rare native plant. Our 13 post 2009 records are scattered over six hectads, SO76, SO93, SO94, SO95, SP05 and SP13. ten of the records by Bert Reid, two by John Day and one by Terry Knight.
Hydrocharis morsus-ranae L. Frogbit
All five recent records of this vulnerable (VU) species were from Grimley Brickpits centred on SO840608. With such similar records I will only describe the most recent, by John Day in 2012. He looked at the Southern Pit, grid SO840607 and SO839607, and found the Frogbit abundant.
Hyoscyamus niger L. Henbane
We have five recent records of a vulnerable (VU) species, an archaeophyte in scattered sites. The records are by A W Reid at Nafford SO943417 in 2010, six plants on bare ground at edge of arable: Keith Barnett at Castlemorton Common SO789397 in 2012, about five in farmyard beside common (record duplicated): Roger Maskew at Bredon Hill in VC33 SO984383 in 2010, about 15: A. Darby at Kemerton in vc33 SO940363, 2010.
Herniaria glabra L. Smooth Rupturewort
This species is of least concern but in Worcestershire had only been recorded by Keith Barnett in 1997, as a small and short-lived patch on the verge of the A38 near Earl’s Croome outside a nursery garden, grid SO862426. On the 22nd September 2014, Bert Reid went to look at same site without any real hope of finding the Herniaria. It wasn’t there and in the nursery garden site there were no plants on sale, but there was a lot of wild-bird seed for sale, often in large commercial lots. I crossed the road and soon noticed a few possible ones. Then at SO863426 I saw a large patch on a disturbed footway edge of the road. I had found it! (01)
Hypochaeris glabra L. Smooth Cat’s-ear
This vulnerable (VU) species is very rarely seen in Worcestershire. The only recent record has been by John Day when he hunted for the species for the BSBI Threatened Plant Survey. The only successful site he found was the MOD Oil Pumping Station, Titton, SO822699. In July 2012 he found several flowering plants in mown sandy grassland.
Jasione montana L. Sheep’s-bit
We have three recent records (two sites) for this vulnerable (VU) species. In 2010 Bert Reid found several at Devil’s Spittleful & Rifle Range N.R., SO8074. In 2012 Brett Westwood saw them on Wilden Motorcycle Scramble Field, SO825728. In 2013 these two recorders joined the Worcestershire Recorders field meeting at Devil’s Spittleful, & Rifle Range N.R.
Juncus conpressus Jacq. Round-fruited Rush
This vulnerable (VU) species has 22 post-2009 records, so many because of the BSBI Threatened Plant Survey. There are ten tetrads in seven hectads so I have selected just one site (Worcester and Birmingham Canal) in 2011. These 12 records were shared equally between John Day and Bert Reid, with John Day recording from SO925630 to SO951670 and Bert Reid from SO914588 to SO917593. The two recorders reported the results slightly differently: both described the habitat as short trodden grass on the towpath, but the plant counts were less comparable. John Day reported numbers of plants or colonies such as 100-200, several colonies or frequent while Bert Reid tried to make more or less accurate counts totalling about 400 plants. It appears that there must be more than 700 plants along that stretch of the canal.
Lithospermum arvense L. Field Gromwell
This endangered (EN) species has four recent records. In2010 and 2011 R.Maskew found several scattered on Bredon Hill above Westmancote, SO946380: in 2011 J.J.Day & A.Darby found it on Kemerton Estate in VC 33, SO946380: and in 2012 T.D.Knight discovered a linear colony about 30m long on the grass verge of Shinehill Lane South Littleton SP088461. The two R.Maskew records are attributed to the wrong vice-county (should be VC 33).
Moenchia erecta (L.) Gaertner, Meyer & Scherb. Upright Chickweed
We have four records of this vulnerable (VU) plant between 2011 and 2013. The 2011 and 2012 records are both by K.Barnett from Malvern (Poolbrook) Common SO788445 & SO789445 and report several small patches in flower on short turf. The 2013 records are both by Dr R.Carter, at Broad Down Malvern Hills near Clutters Cave, SO762393 and at British Camp Malvern Hills, SO765395, no extra details available.
Myosurus minimus L. Mousetail
There are three 2011 records for this vulnerable (VU) plant. At Eckington Ham, SO923426, AW.Reid found three plants by the gateway to the meadow. At Cooks Hill (lane) Wick, SO966469, AW.Reid found two plants in field entrance to pasture, just past north end of lane. At Lower Smite Farm, SO891594, J.J.Day counted 100+ plants between gate & Pylon.
Neottia nidus–avis (L.) Rich. Bird’s-nest Orchid
We have two records for this vulnerable (VU) plant. In 2010 A.W.Reid found six small plants in Tiddesley Wood, SO927457. In 2011 R.Havard & J.Grantham found several plants in Park Wood Mathon, SO763442.
Oenanthe fistulosa L. Tubular Water-dropwort
We have six post-2009 records for this vulnerable (VU) plant. They are 2010 A.W.Reid, Eckington Ham, SO923426. 2011 J.J.Day, Bradley Green, SO996604, 100-200 plants in wet meadow Glyceria swamp. 2010, A.W.Reid, Avon Meadows Pershore, SO9546. 2010 A.W.Reid, Meadow by Lench Ditch, SO975467, locally frequent. 2010 A.W.Reid, Pinvin Roughs, SO949494, several in pool and ditch. 2011 A W Reid, Pool west of Phepson Farm, SO936599, one plant in edge of pool,
Orobanche rapum-genistae Thuill. Greater Broomrape
The most recent record for this vulnerable (VU) plant was in June 2011was at Birchfields, Rochford, SO645669, three flowering spikes and 16 dead spikes from 2010 were seen under broom east of the pool. There were many people attending the field meeting: J.Bingham (who I think first spotted the plants), J.J.Day, B.Westwood, D.Scott, A.W.Reid, G.H.Green et al.
Pedicularis sylvatica L. Lousewort
We have five post-2009 records for this vulnerable (VU) plant. The most recent was in July 2012 at Monkwood Green, SP801601, where J.J.Day found a small colony in a slight hollow with three flowering plants.
Pyrola rotundifolia ssp.rotundifolia. Round-leaved Wintergreen
This is a re-find of the 2009 vulnerable (VU) plant record at Top Barn Farm Gravel Pits, SO835616. J.J.Day’s comment is “Still present in willow scrub: some signs of spread since 2009”.
Ranunculus arvensis L. Corn Buttercup
This endangered (EN) species has long been a specialty in SE Worcestershire. Between 2010 and 2013 we have 13 records: seven tetrads in six hectads. The main centre of the distribution is Naunton Beauchamp parish, SO9545 where plant hunters often gather.
Ranunculus flammula L. Lesser Spearwort
Another endangered (EN) Ranunculus! This time we have nine records in six hectads: SO73, SP74, SO86, SO93, SO94 and SO95. The distribution is more west and central and the habitats are much more in woodland and damp heathland than R.arvensis, that prefers arable. I am sure that R. flammula normally occurs in small quantities whereas R. arvensis is sometimes in hundreds.
Scandix pectin-veneris L. Shepherd’s-ineedle
This endangered (EN) plant has been regularly monitored. Between 2010 and 2013 only two sites have produced any plants. The main site is Naunton Beauchamp c.SO9652, and that accounted for all but one of our seven records: counts here varied from one to 15. The other site is Hawthorns Farm, SO7730. This used to regularly produce one or two plants, but the Scandix disappeared until 2013, when a single plant was found.
Scleranthus annuus L. Annual Knawel
This endangered (EN) plant has been recorded seven times between 2010 and 2013. R.Maskew checked the Martley Scar site, SO7459, in 2010 and 2011 and found “few” and “not much”. B.Westwood looked at Palmers Hill Pasture, SO8980 in 2010, and Wilden Motorcycle Scramble field, SO8272 in 2012, without indicating quantities. J.J.Day recorded in Hartlebury Common, SO8170, in 2011 and found the plant “frequent in open heathland parts of Lower Common”. In 2012 P.G. Garner & J.Grantham found “a few”on Hangmans Hill. SO7638. Still on the Malverns (Pinacle Hill), in 2013, Dr R. Carter “over 300”.
Sorbus domestica L. Service-tree
Although listed as critically endangered none of ours are directly native. Our recent checks have been in Broadway, SO1037, Croome Perry Wood, SO9045, and Areley Castle, SO7680.
Spergula arvense L. Corn Spurry
This vulnerable (VU) plant has certainly diminished over recent years, but is still widespread in small quantities. Since 2010, we have17 records in 12 tetrads spread over seven hectads. Almost all the records are from arable, and changes in farming can rapidly make dramatic population changes.
Stellaria palustris Retz. Marsh Stitchwort
Both recent records of this vulnerable (VU) species were from Grimley Brickpits centred on SO840608. The most recent was by John Day in 2012. He looked at the Southern Pit and estimated 100-500 plants in small numbers along the whole length of the marginal reedswamp.
Torilis arvensis (Hudson) Link. Spreading Hedge-parsley
This endangered (EN) arable plant has survived a bit better in Worcestershire than elsewhere. In 2010-2013 we still recorded in seven monads: SO7731, SO7831, SO8241, SO8556, SP9942, SO9051 and SO9254. The number of plants was less than ten in all but one site. The exception was near the Wychavon Way in Netherton Parish, SP9942. Here there were hundreds of plants along the edge of an Oat field, in both 2010 and 2011.
Trifolium fragiferum L. Strawberry Clover
I almost feel I own this vulnerable (VU) plant. Of the 26 recent records, I found 20 of them. The plant’s Ellenberg indicators show that it is normally found in well lit, rather damp, weakly basic, fertile soil, a perfect description of SO94 and SO95, where I and T.fragiferum live and thrive.
Table of locally infrequent near threatened species
| Euphorbia exigua L. | Dwarf Spurge |
| Cyperus longus L. | Galingale |
| Cichorium intybus L. | Chicory |
| Anthemis cotula L. | Stinking Camomile |
| Briza media L. | Quaking-grass |
| Calluna vulgalis (L.) | Hull Heather |
| Geranium sanguineum L. | Bloody Crane’s-bill |
| Geranium sylvaticum L. | Wood Crane’s-bill |
| Glebionis segetum (L.). | Corn Marigold |
| Inula helenium .L. | Elcampane |
| Knautia arvensis (L.) Coulter. | Field Scabious |
| Lathyrus linifolius (Reichard) | BaesslerBitter-vetch |
| Lepidium campestre (L.) R.Br. | Field Pepperwort |
| Narduus stricta L. | Mat-grass |
| Nepeta cateria L. | Cat-mint |
| Oenanthe lachenalii C.Gmelin | Parsley Water-dropwort |
| Onybrychis ciciifolia Scop. | Sainfoin |
| Ononis spinosa L. | Spiny Restharrow |
| Papaver argenome L. | Prickly Poppy |
| Polygala serpyllifolia | Heath Milkwort |
| Potentilla argentea L. | Hoary Cinquefoil |
| Potentilla fruticosa L. | Shrubby Cinquefoil |
| Salvia verbenaca L. | Wild Clary |
| Silene flos-cuculi (L.) . | Ragged Robin |
| Silene noctiflora L. | Night-flowering Catchfly |
| Solidago virgaurea L. | Goldenrod |
| Succisa pratensis Noebch. | Devil’s-bit Scabious |
| Viola canina L | Heath Dog-violet |
| 01. Herniaria glabra. Harry Green |
Worcestershire Record | 37 (November 2014) page: 51-53 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 7-8 | Worcestershire Biological Records Centre & Worcestershire Recorders
Discovery of Breeding Colony of Ivy Bee Colletes hederae, in Stourport-on-Severn
Jane Scott
Speedy Haircut leads to Discovery of Colletes hederae, Ivy Bee Colony
Since the discovery by Brett Westwood of one solitary Colletes hederae in a garden in Wolverley in 2013, the hunt has been on to find evidence of a breeding colony of these very attractive solitary bees in Worcestershire. Brett gave some interesting background information on the species (Westwood 2013). Geoff Trevis had issued an early ‘look out for this bee’ in 2011 when it had reached Gloucestershire (Trevis 2011).
On 2nd October 2014, husband David’s visit to the barber in Stourport-on-Severn was unusually brief, leaving him time to check a small area of short sloping grass at the edge of a District Council car park before his free parking expired. We had seen odd solitary bees and wasps earlier in the year showing interest in this 45° south facing slope on very light sandy soil, typical of this area.
Although the weather was not ideal, being rather overcast and cool, there were some sunny spells and it was during these that it became apparent that the slope was being used by what could only be Colletes hederae. We were familiar with the species from late holidays in Cornwall and Isle of Wight, when we had seen many individuals and found breeding colonies.
The following day David, myself, Rosemary Winnall and Mick Blythe met at the site in the hope that Rosemary would be able to get some good photographs and the rest of us would be able to catch one or two bees for formal identification by Geoff Trevis, County Recorder for Aculeate Hymenoptera. The day was reasonably sunny, which was fortunate as the number of bees flying definitely reduced whenever it became dull. They seemed to prefer the lower half of the slope which was three to four metres in total depth and the length of the whole grass bank was about 40 metres. We estimated that there could be up to 200 individual nest holes as these were not always as obvious as we at first thought. There were some sizeable patches of flowering ivy in the general area but we did not find any C. hederae using these, although as some was clothing quite high walls the bees could well have been using the sunnier sides which were out of sight.
Rosemary made contact with Wyre Forest District Council to draw their attention to this important find and to try to ensure that the current management of periodic close mowing of the site continues. They have confirmed that there are no plans to do anything more to the site but with much of the actual work going out to contract, the most we can do is keep our fingers crossed.
No other sites were found in 2014, so the hunt for more sites will have to wait until later in 2015. If there is anything to be learnt from this article, it surely must be that it’s always worth giving even the most boring looking area a second glance as it might hold a pleasant surprise!
Acknowledgements
Thanks to Rosemary Winnall for providing the photographs to accompany this article.
Reference
Westwood, B. 2013. – Ivy Bees Colletes hederae in Worcestershire. Worcestershire Record, 35:
Trevis, G. 2011 Alien bee Colletes hederae reaches Cheltenham . Worcestershire Record 31:16-17.

01. Colletes hederae bee bank, Stourport. Rosemary Winnall

02. Colletes hederae bee bank, Stourport. Rosemary Winnall

03 Colletes hederae bee bank, Stourport. Rosemary Winnall

04. Colletes hederae in Stourport. Rosemary Winnall

05. Colletes hederae in Stourport. Rosemary Winnall
Worcestershire Record | 37 (November 2014) page: 7-8 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 18-19 | Worcestershire Biological Records Centre & Worcestershire Recorders
Platycis minutus Net-winged Beetle at Woodbank, Astley Burf
Jane Scott
During 2012 individuals of Platycis minutus a Nationally Notable beetle were seen in Worcestershire and reported in Worcestershire Record in 2012 & 2013. These were all singletons and seen in the north west of the county, three in Wyre Forest area and one at Astley Burf. Rotting beech is frequently mentioned as a favoured wood but birch is also used and we certainly have plenty of this at Woodbank, including quite a lot of fallen timber which we leave in situ to rot down wherever possible.
On 28th August 2014, two beetles, both about 5 mm long were seen at Woodbank, one on nettles and the other showed great interest in the corner of one of our raised beds in the vegetable garden. I had read that this species is known to cluster, so the following day I checked around the nettles and found none but on searching around the raised bed I found seven individuals, one or two noticeably larger at about 8 mms. Some were tucked well down the inner side of the raised bed but others were sitting on top of the edge whilst a couple were found at the top end of longer grass just outside the bed. This corner of the raised bed is by far the most shaded and gets the sun for only a short while at this time of the year. Taking a closer look I found that the wooden boards were showing distinct signs of rot on the inner side at and below soil level and I assume it was this that had attracted them to the spot, although I have no idea what timber was used in the manufacture of the boards. (01, 02).
I continued to check them most days from 25th August until 7th September and they remained in the same spot, either just walking slowly around or resting and showing no sign of aggression if, as occasionally happened, their paths crossed. During this time, on 1st September there was a mated pair confirming my view that the larger ones were female (03). By the 10th there were only two in evidence, both males but we did see what appeared to be a female sitting on the back porch 50 metres away. The following day a male and female were seen at the raised bed, whilst on 12th, only one at the raised bed but another was found by David sitting near the top of our large conifer hedge some 100m away. None were around the original spot on the 13th but we did find a female inside the back porch.
On the 14th September the magnificent seven were all back at the raised bed and this time there was a lot of activity with a female walking around with three or four males on top, (04) one of which appeared to have sustained damage to both the wing and wing case during this encounter. I checked later in the day and the female, still with her entourage attached, had moved to the inside of the boards and appeared to be trying to tuck herself well into the area of the rotting wood. I watched for some time but did not see any evidence of egg laying.
After all the excitement on the 14th no beetles were seen the following day and between the 15th to 27th September the occasional male appeared at the raised bed. This was not always the same individual, as I saw the beetle with the damaged wing case once or twice but at other times there was no evidence of any damage on the individual seen.
Over the five week observation period it was interesting to note they could be seen at anytime from early morning to dusk and, on days when I was able to check, they were in evidence throughout the day rather than just appearing occasionally. The Net-winged Beetles have a reputation for being difficult to find as they have a relatively short season as adults but they are known form clusters and I think I was fortunate to have had the opportunity to view them with relative ease.
References
Bingham, J. 2012. Invertebrate Records from Worcestershire 2012 – Worcestershire Record 33:7-9.
Brown, A. 2013. Coleoptera of note found in the Kidderminster area 2010-2013. – Worcestershire Record 35:25-27.
Brock, P. D. A comprehensive Guide to Insects of Britain & Ireland. Pisces Publications
Watford Coleoptera Group: www.wcg.org.uk.

01. Platycis minutus 11.09.14. Jane Scott.

02. Platycis minutus 31.08.14. Jane Scott.

03. Platycis minutus mating cluster 01.09.14. Jane Scott.

04. Platycis minutus mating cluster 14.09.14. Jane Scott
Worcestershire Record | 37 (November 2014) page: 18-19 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 9-10 | Worcestershire Biological Records Centre & Worcestershire Recorders
A Notable B ‘bird dropping’ — an anecdote
Martin Skirrow
Despite a lifetime of fascination with the natural world, I never cease to be astonished and enlightened by it. On 11th April 2014 I saw what I took to be a small cylindrical bird dropping exposed on a slate ledge at the foot of a building on the farm where I live in Berrow (SO777339). I was on the point of ignoring it when I noticed that it had a distinct symmetry. On picking it up I found it to be hard and dry without any obvious projections or excrescences. As my insect room was nearby, I took it in for examination, but as I placed it under the microscope I thought “What on earth am I doing playing around with this bit of obvious bird excrement!” But then came revelation: a dorsal suture, two eyes and a snout; and underneath, legs and antennae tightly folded in their grooves and flush with the body. I presumed it was long dead; but these appendages were movable with a seeker, so perhaps it had not been dead long. Five minutes later it walked off and sought exit from its container!
It appeared to be a large weevil (01) and initially I thought it was Platystomos albinus, but Harry Green, without seeing it, raised the possibility of it being Platyrhinus resinosus (Anthribidae), which is indeed what it turned out to be. I was initially misled by an on-line photograph of P. resinosus wrongly labelled Platystomos albinus (beware of unsubstantiated postings!). Larvae of P. resinosus have been found in the Cramp-Ball fungus Daldinia concentrica, and its common name is Cramp-Ball Fungus Weevil, but it has also been called King Alfred’s Cakes Weevil after the popular name for the fungus. The beetle appears to be dependent on the occurrence of the fungus, which grows on dead wood, especially beech and ash. There is no beech at the farm, but many mature ash with dead wood both on trees and fallen. The fungus has been seen regularly, and our policy to leave such dead wood in place.
The species is classed as Nationally Scarce Notable B with a distribution mainly from the Severn valley through the Midlands and north to Yorkshire. In Worcestershire it has been recorded almost every year since 1991, mostly from Bredon Hill, Tiddesley Wood, and Mill Rough near Drakes Broughton, all areas where there is old woodland (02). Further away, several beetles were found by Alan Brown in Springfield Park in the Kidderminster area in June 2011 (Brown 2013). The present record is also an outlier, but in the far south west of the county (grey square on the map). It is likely that the beetle occurs in other areas where there is ample dead wood and fungus.
Acknowledgement
My thanks to Simon Wood for extracting records of Platyrhinus resinosus from the Worcestershire Biological Record Office’s database.
Reference
Brown, A. 2013. Coleoptera of note found in the Kidderminster area 2010-2013. Worcestershire Record. 35:21-27.

01. Platyrhinus resinosus Cramp-Ball Fungus Weevil (Anthribidae). Martin Skirrow
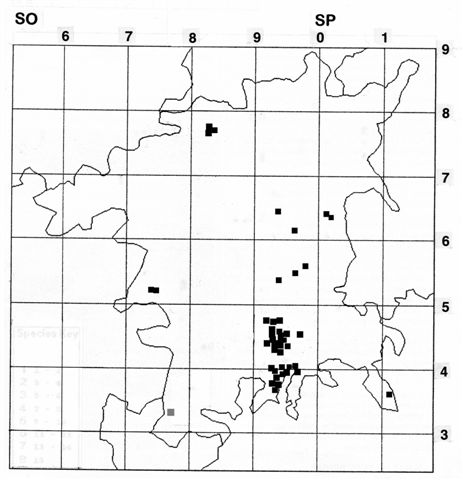
02. Distribution of Platyrhinus resinosus in Worcestershire. Martin Skirrow.
Worcestershire Record | 37 (November 2014) page: 9-10 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 33-35 | Worcestershire Biological Records Centre & Worcestershire Recorders
Dittander Lepidium latifolium L. at Droitwich
Geoff Trevis
Introduction
Dittander Lepidium latifolium is a rather unprepossessing, perennial plant with fleshy leaves and an inflorescence of small white flowers, which grows to a height of about 1.5m. It belongs to the halophyte (salt tolerant) group of plants. This does not mean it requires salty conditions to grow but rather that it has a competitive advantage in salt marshes. It is widely distributed in Britain though away from its natural locations it is not common. Its alternative name, Pepperwort, gives the clue to the distribution. Its leaves are said to be an excellent addition to salads and it may, therefore, have been planted as a culinary herb.
In Worcestershire it is very uncommon, being restricted to the Salwarpe valley, mainly on the banks of the river Salwarpe, between Drotwich Spa and Hawford. In times past the area south of Droitwich Spa to Salwarpe was a salt marsh, resulting from the Droitwich salt industry, which contained many halophytes in addition to Dittander. John Amphlett’s contribution on botany in the Victoria History of the County of Worcester simply states that among other halophytes L. latifolium grows abundantly by the Salwarpe at Droitwich.
History
The first record of dittander in Droitwich was made by Rev. J.H. Thompson in 1852 (formally reported in 1853) when he says “Last October, I had the pleasure of adding to the Worcestershire Flora, L. latifolium, which I found close to the river Salwarpe, where it is crossed by the Wolverhampton Railway near Droitwich. I first saw some patches of it on the recently-formed railway embankment; but on further examination I detected some old plants in a muddy place by the river-side, where, perhaps, they have been growing secluded and unnoticed for a long time”.
Edwin Lees (1854) confirms this when he says that dittander grew “on the banks of the Salwarpe at Droitwich, just where the railway to Stoke crosses the river” and that it was discovered by the Rev. J.H. Thompson “who pointed out a large patch of the plant to me there in 1852”. At this point I cannot help but take a slight diversion to give a flavour of Lees’ description of the site, “pepperwort was found at a spot where the river, loaded with garbage, slowly emerges from among the sooty chimneys of murky Droitwich”! Interestingly, there is a slight difference between these two reports. Thompson says he found it by the bridge where the Wolverhampton Railway crosses the river whilst Lees says it was by the bridge where the Stoke line crosses the river – these are, I suspect, different branches as the Wolverhampton line would be the one going to Stourbridge whilst the Stoke line is that going to Birmingham via Bromsgrove. In another of his books, written in 1867, Lees again notes L. latifolium at Droitwich with the description “On inspecting the locality, though the plants on the railway embankment appeared of recent origin, yet old seedy plants by the side of the dingy Salwarpe looked as if they had grown grimy there for many a year”.
The Worcestershire Naturalists Club made many visits to the Salwarpe Valley, walking the canal and river to Hawford and making small diversions to interesting local sites including Westwood Park. In 1880, their Transactions record that “The very uncommon Broad-leaved Pepperwort (Lapidium latifolium) was, however, found in its old habitat on the railway embankment near Droitwich”. A similar record is found from a visit in 1934 where the plant was found “near the canal bridge at the old wharf” and in 1943 the first record near to its current location at Briar Hill Coppice, when “several nice examples” were noted on the approach to Briar Mill (this is near Droitwich High School and should not be confused with the reserve at Briar Hill). In 1951 Fred Fincher wrote an article for the Transactions of the Worcestershire Naturalists Club about the maritime plants with records between Porter’s Hill Farm and Droitwich, growing along side the river, included Dittander.
A report in 1954, whose origin I have been, so far, unable to attribute notes a recommendation by F. Fincher that the salt marsh area should be scheduled as a Site of Special Scientific Interest (SSSI). There is another rather interesting record from 1967 saying that a drainage scheme implemented by the Borough Surveyor affected part of the site and the Trust (which?) arranged to move individual plants of Dittander to another, safer part of the site. The SSSI designation came into force in 1972 following a further report by Fred Fincher in 1970 that records “along the banks of the Salwarpe there is also a fair amount of Dittander, one of the most interesting halophytes for which the area was noted. About seven main sites still remain and it appears in no danger”. It is, however, difficult to pin down exactly where the plants were. Since 1972 drainage, cultivation and dumping of top soil, combined with the demise of the salt industry gradually reduced the salt marsh and the SSSI was de-scheduled in sections.
Large stands of Dittander were photographed near Briar Hill Coppice Reserve in July 1980 growing adjacent to the Salwarpe (01, 02, & 03). The pictures are converted from old Kodak transparencies.
A report of a river corridor survey of the Salwarpe in 1992 to National Rivers Authority notes that “Lepidium latifolium (Dittander) is a common component of open stretches of tall herb habitat along the banks of the Salwarpe downstream of Droitwich” and its positions are indicated on the sketch maps of each section surveyed (Castle 1992).
Roger Maskew (2014) also records several references to Dittander in the literature. In particular, he notes “In what is now accepted in Preston et al. (2002) and Stace et al (2003) as its most inland British native site, Dittander is at present scattered and locally frequent along the banks of the Salwarpe from Hawford (SO8460) to Netherwich (SO8963) but apparently not seen further east along the river in SO96 since the 1980’s.” Most interestingly he also records a large stand over some 10 m on the slip road of the M5 at Wychbold (SO9165) in 2009!
The Source of Dittander
The origin of Dittander at Droitwich which, as noted earlier, appeared some time before 1852, is uncertain. Lees (1854) and Thompson quote the idea of Prof. James Buckman that the presence of halophytes around Droitwich, and some other places along the Severn flood plain, “affords good evidence that marine conditions once prevailed along the greater part of the Severn, and that the marine waters were far wider than the reach of even the floods of our day”. However, it was also suggested that the plants arrived with barges coming up the canal from the Severn estuary, particularly from Bristol. Amphlett and Rea (1909) take another view, that the plant is an escape from local cultivation. The evidence for transport from Bristol or for garden escape is not strong either way but I would think that its survival over a long geological period, possibly since shortly after the last ice age, is less likely.
The current situation at Droitwich and Droitwich Community Woods
My own involvement with the site began in about 1984 and I have known Dittander growing quite profusely on either side of Ombersley Way where the road crosses playing fields and the Briar Hill Coppice reserve (now part of Droitwich Community Woods). Records held by WBRC show its presence in the Salwarpe Valley until 2001, including occasional records along the canal and, indeed, I found a single plant close to the railway bridges over the canal only this year. Nonetheless, over the last few years the number of plants has gradually declined until it became extinct in all its usual Droitwich sites except for some vigorous growth among unmanaged patches of nettles Urtica dioica and brambles Rubus fruticosus agg. close to Ombersley Way on open space belonging to Wychavon District Council, but which is not part of the reserve. I thought this was the end of this important aspect of the Community Woods but more recently I was surveying part of the reserve at the north end, called Appler’s Piece, which has been unmanaged for many years, and was delighted to find good stands of Dittander growing along the banks of the Salwarpe and further into the middle of the area. The vegetation again is dominated by nettles with some brambles, hogweed Heracleum sphondylium, hemlock Conium maculatum, teasel Dipsacus fullonum and some quite striking stands of small teasel Dipsacus pilosus.
The question is, why did the plant persist in some unmanaged areas whilst it died out in other similar unmanaged patches where it had previously been abundant? I can think of no obvious answer to this.
I have found several references to the culinary use of Dittander and its cultivation. In essence it appears that this plant does indeed produce edible leaves which have a hot taste comparable to nasturtium and horse radish and it responds well to being pruned when it produces small, even tastier leaves. The down side is that its root system is even more vigorous than the parts above ground and it produces roots which spread everywhere and send up new shoots in abundance, comparing well with ground elder Aegopodium podagraria and couch grass Elytrigia repens!
This being the case, one can understand why it has persisted so well in some places but does not suggest any reason why it has died out in others. Do we need to introduce some management to ensure its continuing survival? If so, should this simply involve strimming every few years to check nettles etc., should we harvest some plants to encourage new growth? Should we perhaps harvest seed and try to re-establish it in its old haunts? At the moment I am not sure and perhaps we will have to try some limited experiments on small patches to see what happens. Anyway, it is at least good to know that this rare plant continues to thrive at present and has not, as first thought, become extinct at Droitwich Community Woods.
References:
Amphlett, J. & Rea, C. 1909. The Botany ofWorcsetershire. Cornish, Birmingham.
Amphlett, J. 1901. Phanerogamia (Flowering Plants) in The Victoria History of the County of Worcester, Volume 1. Dawsons, London.
Castle, G. 1992, River corridor survey Salwarpe, Worcestershire, June 1992. Unpublished report to National Rivers Authority.
Fincher, F. 1951 Worcestershire Maritime Plants. Transactions of the Worcestershire Naturalists Club 1950/51 10 (4) p. 265-269.
Fincher, F. 1970. A report believed to have been written for the Nature Conservancy Council.
Lees, E. 1856. Pictures of Nature in the Silurian Region Around the Malvern Hills and Vale of Severn: Including Incidental Excursions with the Malvern and Worcestershire Clubs: and Notices of the Natural History, Pictorial Scenery, Botany, Geology, Customs and Superstitions of Many Interesting Localities in Worcestershire and Herefordshire. Lamb, Malvern.
Lees, E. 1867. The Botany of Worcestershire, or the Distribution of the Indigenous and Naturalised Plants of that County, with Descriptions of the most Remarkable Localities for Botanical Observation, Including Sketches of the Physical Geography of Worcestershire in Four Botanical Districts, and a Tabulated Arrangement of Plants, showing their Frequent or Rare Occurrence in each Division. Worcestershire Naturalists’ Club, Worcester.
Lees, E., 1854. Transactions of the Worcestershire Naturalists Club, August, page 11.
Maskew, R. 2014. The Flora of Worcestershire. Published privately.
Preston, C.D., Pearman, , D.A. & Dines, T.D., Eds. 2002, New Atlas of the British and Irish Flora, Oxford University Press, Oxford.
Stace, C.A., Ellis, R.G., Kent, D.H., & McCosh, D.J.. 2003, Vice-county Census Catalogue of the Vascular Plants of Great Britain. Botanical Society of the British Isles, London.
Thompson, J.H. 1853, A note on the Worcestershire species of Lepidium. Phytologist, 4:970.
Transactions of the Worcestershire Naturalists Club 1934 p. 104
Transactions of the Worcestershire Naturalists Club 1943 p. 34

01. Dittander Salwarpe Droitwich July1980. Harry Green.

02. Dittander Salwarpe Droitwich July1980. Harry Green

03. Dittander Salwarpe Droitwich July1980. Harry Green
Worcestershire Record | 37 (November 2014) page: 33-35 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 31-32 | Worcestershire Biological Records Centre & Worcestershire Recorders
Kites, Ravens and other raptors of the Broadway, Worcestershire, area, March to August 2014
Mark Turner & Christine Turner
Two Kites take up residence in the Broadway area; Ravens enjoy breeding successes; Peregrines raise young. The grapevine is murmuring!
References to Kite hereafter means Red Kite Milvus milvus and Buzzard the Common Buzzard Buteo buteo.
30th March. 2 Kites with high wheeling Buzzards during the afternoon at Bourton-on-the-Hill/Batsford Park, SP1833.
1st April. One Kite circling low over Evesham Road, Broadway, SP086385, mid-afternoon.
2nd April. Two Kites flying to the escarpment via Leamington Road, Broadway, SP100377-108379, late afternoon.
3rd April. Two Kites sought woodland refuge in fog late afternoon, SP108379.
21st April. One Kite southwest of Broadway Gravel Pit in a thermal stack also comprised of 1 Sparrowhawk (uppermost bird) and 5 Buzzards, SP085377.
28th April. Two Kites (single birds) going southwest passed Broadway Gravel Pit, SP087379.
30th April. One Kite seen from home at Bridgemans Close, SP102380, soaring high over Bibsworth/Sandscroft/escarpment fields, had symmetrical moult of inner primaries.
2nd May. One Kite west of Broadway Gravel Pit hanging aloft before a mobbing by corvids forced it down.
4th May. Seen from back garden at home one Kite high overhead was gliding very slowly south.
5th May. One Kite high above Broadway Gravel Pit with a Buzzard after midday.
12th May. One Kite southwest of Broadway Gravel Pit over allotments.
13th May. One moulting Kite swooping low around housing estates of Lime Tree/Bibsworth/Sandscroft Avenues, SP102380, at 15.40 hrs and picking up a mobbing by three Jackdaws. As is often the case an accompanying Buzzard was nearby.
16th May. One moulting Kite at 18.20 hrs circling over towards the village bypass to the northeast of home in Bridgemans Close.
17th May. One non-moulting Kite seen from home again to the west at 16.15 hrs. This was followed by a Peregrine at 17.20 hrs circling high to the west drifting off north.
2nd June. One Kite after midday circling over allotment fields to the west of Broadway Gravel Pit mobbed by a Jackdaw.
5th June. One Kite just after mid-morning again over the allotments opposite Broadway Gravel Pit.
19th June. One Kite moulting outer primaries and tail, briefly in company with a Buzzard hung around over Sandscroft and Bibsworth Avenues then headed off southeast just after 15.30 hrs.
1st July. Watching with increasing excitement from our back garden from 14.00 hrs, raptor activity intensified in a short space of time. Two low-circling Buzzards were soon followed by a Kite from the direction of Leamington Road and the adjacent infants’ school. The Kite’s central tail feathers were missing. There then came an adult Peregrine from the same direction which reached us overhead before turning and speeding away. Buzzard numbers reached five or six plus a female Sparrowhawk carrying prey.
Earlier during the morning we were treated to a prey-carrying Peregrine at Ford, Gloucestershire, and a pale form Buzzard at Broadway Tower plus a bonus of two juvenile Ravens in a tilled field.
7th July. One Kite at 08.30 hrs seen from home circled to the north then off towards the escarpment. Also late morning seen from Broadway Gravel Pit, One Kite to the south over the village.
8th July. One un-moulted Kite to the west of Broadway Gravel Pit at 10.30 hrs.
27th July, observing from home. After a fresher than usual start from 10.30 hrs it was warming up noticeably and a build up of cloud evidently produced thermalling opportunities for local birds. Small groups of Ravens and Buzzards began to appear three or four birds at a time, but very quickly on the scene there appeared an adult Peregrine fairly low over our estate soaring up virtually overhead then drifting back towards the escarpment to the east. On reaching the hill it stooped on an emerging Buzzard from above before circling up ever higher to join its mate. Christine’s superior hearing picked out contact calling between the falcons.
After a short space of time we suddenly had a return flypast by a Peregrine and I am sure the attraction here is a healthy population of Swifts and House Martins let alone Jackdaws that number 40 or 50 pre-roost. The highly entertaining Swifts and Martins are great for alerting us to the presence of a threat, though occasionally it may just be patrolling Jackdaws or Magpies. Interestingly I’ve noticed Swifts will chase and mob individual Jackdaws innocently passing through the neighbourhood.
A little after 13.00 hrs I noticed a Buzzard in a very assertive display over hillside woodland territory (again observed from our garden), it was undulating, stalling, then plunging earthward. At this time of year it is said to be a sign of parent birds attending well-grown young and woe betide any bird or anyone for that matter coming near. Whether the Buzzard was responding to the Peregrine presence I don’t know, but the falcons were still patrolling at this time. Around 13.30 hrs one of the adult falcons drifted away from the ridge to soar over our estate again before returning quite hastily.
On a general note since Peregrines have become year round residents and breeding successfully, I have noticed a decrease in sightings of Sparrowhawks, Kestrels and Hobbies locally. Whether there is a connection between these facts is open to discussion, but certainly I have seen Sparrowhawks and Kestrels mainly during earlier and later hours than for Peregrine; perhaps avoidance for them is the best policy. However, I have a greater concern that Sparrowhawks are genuinely in decline, but find it difficult to come up with a reason other than possible persecution.
Hobbies have been a real success story, but 2014 has not been as we have come to expect in recent years from these local Hirundine hunters. However, back to the afternoon of Sunday 27th July and at 15.20 hrs patience was rewarded. From my back garden viewpoint a Hobby travelling west away from the escarpment was in hot pursuit of a singled-out House Martin. This unsuccessful attempt was followed by the slender falcon regaining height and continuing on towards the village centre.
The next Peregrine flypast was no less awesome just twenty minutes later passing directly overhead whilst three Buzzards hanging into the wind over the hillside wood looked more like Lancaster bombers by comparison. The Peregrine pair appeared together to the west of my position at 15.55 hrs gliding round and round with wings held absolutely flat.
28th July. At 15.15 hrs it was the turn of the Buzzards to show off in our airspace. Having seen nothing raptor-wise I found myself distracted by a glider using a thermal directly overhead, beneath a bank of storm cloud. Before many minutes had passed three Buzzards latched onto the same thermal beneath the glider and then with primaries angled rearward all three birds filed away in a line towards the wooded hill to the east. However, the one at the tail-end picked up speed and launched an attack on the others. This was never going to be a happy alliance at this time of year; a territory holding adult will always be on the look out for approaching intruders.
At long last 15.50 hrs brought a female Sparrowhawk from the south heading in a straight line over the avenues in a very slow glide with occasional wing flicks to maintain momentum. Turning east over arable fields the hawk picked up speed in a shallow angled descent.
30th July. A brief note that our Peregrine pair were hunting high over Sandscroft Avenue and the arable field adjacent to the bypass around 17.00 hrs.
31st July. Also briefly, a note on a soaring and half-barrel rolling Raven projecting its far-carrying foghorn-like croak high above the residential estate and Broadway Hill late morning.
1st August. We were once again treated to a back garden flypast by a Hobby casually checking out the Avenue’s House Martins at 09.40 hrs. It seems odd how as Kite watching waned all else are coming to the fore, but I feel confident we haven’t seen the last of the great fork-tailed ones. To be continued.

01. Peregrine by Mark Turner.
Worcestershire Record | 37 (November 2014) page: 31-32 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 27 | Worcestershire Biological Records Centre & Worcestershire Recorders
Armadillidium pulchellum: A new pill-woodlouse for Worcestershire
Brett Westwood
On May 19 2014, I visited Kingsford Country Park on the Worcestershire /Staffordshire border. This is mainly acid heathland on New Red Sandstone colonised by birch and planted with Scots Pine. Areas of the birchwood have been cleared recently to encourage reptiles and heathland birds.
In a cleared area where Wavy Hair-grass (Deschampsia flexuosa) has recolonized, I turned over a burnt birch log left after a fire. Under it were numerous woodlice (Oniscus asellus) and a smaller creature which looked like a tiny burnet moth larva, about 4mm long and striped in yellow. Only when photographed (01) was I able to see that it was a brightly-patterned pill-woodlouse and one of two local species, Armadillidium pulchellum or the rarer A. pictum. Neither has been recorded as far as I knew in Worcestershire, so I kept the specimen and gave it to John Bingham to photograph. His excellent photos (02 & 03) revealed the bright colours and characteristic features and I was able to send them to Steve Gregory of the British Myriapod and Isopod Group who confirmed the specimen as Armadillidium pulchellum from the chamfered edge to the first pereonite segment.
This species occurs locally in the north and west of the UK and Steve Gregory commented that the Kingsford record bridged a gap between the Welsh and Peak District sites. The typical habitat for A. pulchellum is dry calcareous grassland, though in Devon and parts of Wales it favours overgrown heaths, bracken, stone walls and oak woodland. In southern and eastern England where it is rare, it is associated with heathland often where conifers have been planted. Although it was once thought to be very scarce, records have increased as naturalists become aware of its identity.
It is a very small creature just 2mm diameter when rolled up, but worth looking for in other parts of Worcestershire especially on heaths or in dry open woodland. Careful separation is needed from Armadillidium pictum which is much rarer, but may occur in the north or west of the county.

01 Armadillidium pulchellum at Kingsford. Brett Westwood

02 Armadillidium pulchellum at Kingsford. John Bingham

03 Armadillidium pulchellum at Kingsford. John Bingham
Worcestershire Record | 37 (November 2014) page: 27 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 36-45 | Worcestershire Biological Records Centre & Worcestershire Recorders
An exposure in the Pershore Member of the Avon Valley Formation at February Piece, Allesborough Hill, Pershore, Worcestershire
Paul F. Whitehead
Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH email: paul@thewhiteheads.eu
Introduction
Changes in the fluvial regime of the River Avon during the past 300,000 years or so have resulted in the deposition of Pleistocene sands and gravels traceable under a series of terraces traditionally numbered from one to five in ascending order above alluvium (Tomlinson, 1925). These comprise what is now the Avon Valley Formation. De Ruffignac et al. (1995) established the existence of a further aggradation, the Strensham Member, which has not yet been accommodated within a numbered framework (Table 1). It should be noted that the depositional history below a single ‘terrace’ surface may transcend a range of climates (Whitehead, 1992), a matter now rather more subtly demonstrated within the Pershore Member (Table 1).
Evidence for climate and biota of the Pershore Member were unknown until 1975. In that year contemporaneous plants, molluscs and vertebrates were obtained from sections cut during a road-widening scheme (03) on Allesborough Hill, Pershore (Whitehead, 1989a).
During March 2014 building work commenced at a site at Allesborough Hill, Pershore, known as February Piece (SO9346. 01 & 02), a name deriving from the ameliorating effects of underlying sands and gravels on traditional arable cropping practice. Construction work required the installation of a pipe trench almost four metres deep (02); this sectioned the entire thickness of the Quaternary sediments the visible evidence for which forms the basis of this paper.
| 02. Avon Valley Formation. Location of pipe-trench section A – D through Pershore Member, February Piece, Pershore, Worcestershire, April 2014 in relation to the April 1975 exposure (03). Ordnance Survey grid references for A – SO93744620; B – SO93794621; C – SO93854622; D – SO93894623. |
These relatively high level sediments, some 37 m above modern alluvium, provide some degree of correlation with the release of water from pro-glacial Lake Harrison (Bishop, 1958) which established the modern Worcestershire River Avon. This reversed the directional flow of its fluvial predecessor (Shotton, 1968) leading ultimately to the creation of the Severn-Avon confluence at Tewkesbury. Sediments above the modern river valley on higher ground to the north are glacigenic or glaciofluvial in origin.
| Terrace no. | Member/bed no & name | Above alluvium | MIS | Years BP | Time period |
| Avon No 1 | *Bretford Member | 1m (Lower Moor) | 2 | 12500 | Devensian |
| Avon No 2 | *Wasperton Member | 3m | 2 | 29000 | Devensian |
| Avon No 3 | ***New Inn Member | 8m | 5e | 120000 | Ipswichian |
| Avon No 3 | ***Eckington Bed | 8m | 5e | 120000 | Ipswichian |
| Avon No 4 | Twyning Member | 18m *Lower Bed | 6 | 150000 | ‘Wolstonian’ |
| Avon No 4 | Twyning Member | 21m ***Upper Bed | 7 | 180000 | ‘Wolstonian’ |
| Avon No 4 | ***Cropthorne Member | 19m | 7 | 200000 | ‘Wolstonian’ |
| ————– | **Strensham Member | 29m | 7 | 220000 | ‘Wolstonian’ |
| Avon No 5 | *Pershore Member | 37m | 8 | 270000 | ‘Wolstonian’ |
| Avon No 5 | **Allesborough Bed | 33m | 8 | 280000 | ‘Wolstonian’ |
Table 1. The Avon Valley Formation as at 2014. Chronological sequence of aggradations by increasing age downwards, geological name, surface height above alluvium, marine isotope stage (MIS) and guideline dates (“Years BP” approximation of years before present in part from Lisiecki & Raymo, 2005). The traditional application of the ‘Wolstonian’ stage name requires clarification to accept the range of climatic variation indicated here. Simplified climate and biota: *full glacial **cool temperate ***warm temperate.
Sedimentology
The approximate length of the visible pipe-trench section during April 2014 is shown in 02 with stations A-D indicating where observations were more focussed. The sediments varied in their fine details along the length of the cutting but 04 typifies them and illustrates the use of large retaining frames required to overcome sediment instability. The basal unit consists of a thick layer of unbedded red quartzose sand resting on Jurassic bedrock, unstable in section due to active zones of water transmission (04, 06). These sands, not everywhere equally evident but in places somewhat gravelly basally, are believed to be the fill of channels with changing or variable flow rates. The sand presumably results from seasonal weathering effects in an open exposed landscape. The sand is composed of subrounded to subangular grains with an average diameter (visual scan of thousands) of 0.3 mm; a quartz grain measuring 3 mm diameter was noted together with larger rounded quartzite fragments. The sand grain edges are all more effaced than those of the upper loessic sands (see below). The binding matrix of the sand is ‘dust’ or silt with particle sizes submicroscopic but at least as small as 0.002 mm diameter; this is presumably loessic dust introduced from an open exposed treeless landscape.
Trias-derived coarser gravel dominated by quartzite and flint in a quartzose sand matrix with little local Jurassic content, in places brecciated by ferrocretion (04, 10a & 10b), overlies the red sand unit. These gravels are overlain by finer friable sandy sediments which have the characteristics of loess and attain a thickness of 40 cms in places (04 & 05). These unbedded sediments are composed of interlocking subangular to subrounded sand grains bound by traces of calcareous ‘dust’ or silt. The sand grains vary from 0.09 mm to 0.7 mm diameter, an average diameter being close to 0.3 mm. These may have originated from exposed high ground or plateaux above the catchment and may be of glacigenic origin. The modern land surface is underlain by loams with a significant Jurassic clay content introduced from upslope over a probably extended time period.
Climate of deposition
The upper sands and gravels have been subjected to a range of cold climate processes for which significant visible evidence was found. These include cryoturbation (08) and ice-wedge pseudomorphs or casts, ground contraction fissures subsequently refilled with sediment (07).
| 07. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 8 April 2014. Cryoturbation of ‘loessic sand’ and underlying gravels with intraformational ice wedge cast almost one metre deep. Northern face of pipe trench close to A in 02 SO93744620. Context is visible also at west end of 08. |
| 08. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 8 April 2014. Location of flint scraper (10) in ferrocreted flint, quartz, and quartzite gravel with abundant frequently subangular to angular quartz grains. Northern face of pipe trench at A in 02 SO93744620. Trowel is 28 cms long. |
There is also evidence for the infilling from upslope of surface depressions of possible cryogenic origin with diamicton in which larger clasts were haphazardly suspended in a viscous mobile matrix of finer sediment (09); this process is believed to be broadly contemporaneous with member development but this is not proven.
These features demonstrate that towards the end of aggradation the climate was of full glacial severity with the development of permafrost. This climatic deterioration is held to occupy the maximum cold of Marine Isotope Stage 8 (MIS 8) occupying ‘Wolstonian’ time. The Allesborough Bed (Whitehead, 1989a; Maddy, 1999) is here regarded as marginally older than the sediments of the Pershore Member and contains cool temperate biotic elements.
Geochronological implications for the Worcestershire River Avon valley
If it is accepted that these periglacial conditions mark MIS 8 and that the full glacial sediments of the basal Twyning Bed (Avon No. 4 terrace of Whitehead, 1989a; 1992 actually in Watsonian East Gloucestershire) are pre-Devensian a clear scenario presents itself. The first major episode in the cutting of the modern River Avon consequent to the drainage of Lake Harrison during the ‘Wolstonian’ deepened its valley by 20 m before the Twyning basal bed aggraded in MIS 6 time. The Strensham and Cropthorne members represent halt stages in this process during MIS 7 when sea level rose again; the observations on MIS 7 made by Lang & Wolff (2011) are highly pertinent when attempting to disentangle the finer nuances of River Avon ‘terrace’ climate and biota.
On this basis three ice ages passed whilst the modern River Avon incised to its present level thus according with Bowen (1999) and Lowe & Walker (2014). When Keen (1999) implied a MIS 9 age (followed by van Asperen, 2009) for the Pershore Member it was on the basis of working back from the known ages of the younger terraces; he appears to have overlooked the full glacial biota beneath Avon No. 4 terrace surface at Twyning (Whitehead, 1989a; 1992) with the implication now that the English Midland ‘Wolstonian’ may not require to be moved back in time and that the sequential position of Avon No. 5 terrace is closer to the ‘old’ one (Shotton, 1973). Sutcliffe (1995) did acknowledge Avon No. 4 terrace at Twyning and he too discussed the problematical use of ‘Wolstonian’ as a stage name, a discussion that is still ongoing (Bridle, 2012). It seems to me improbable that the Allesborough Bed at Pershore sensu Maddy (1999), possibly reflecting the demise of the large mammal fauna prior to the onset of full glacial conditions, is significantly different in age to the Pershore Member at Pershore.
In referring to Avon No. 5 terrace Keen (1999) employed the term ‘interglacial’ on the basis of ‘temperate’ biotic elements but these are few and, especially in the light of this new evidence reference to an interglacial is not advisable. The descent to full-glacial could have been rapid. The absence of a full glacial ‘mammoth-steppe megafauna’ in the Pershore Member might, if it is real, be explained by the absence of rich productive floodplain grassland. This is known to have reached its greatest development on two subsequent occasions but only after enough time had been allowed for the river to become well incised into bedrock and create the fluvial regimes that favoured this habitat and its distinctive biota.
Archaeology
Two lithic artefacts recovered from the pipe trench are the only evidence of human culture from the Pershore Member up to now. They are a discoidal scraper (11a, 11b & 11c) of early Middle Palaeolithic affinity worked on coarse far-travelled cherty flint with centripetal removals defining one flat surface (11b). The exact in situ position of the scraper in the quartzose sands and gravels is depicted in 08.
The second artefact is a concave scraper worked on Jurassic fine-grained micaceous siltstone (12a, 12b) found ex situ close to the contact of the gravel and bedrock. The siltstone is uniformly laminar, some four millimetres thick with its long-edge facetted by man; this allows it to rest easily between the index finger and a platform created by another finger of the same hand. Jurassic micaceous siltstones and ironstone were observed near or close to the contact of the sands and gravels with the underlying Charmouth Mudstone. Neanderthal man evidently found that brittle Jurassic siltstone constituted an acceptable resource which is suggestive of local human presence.
Biota
No organic lenses or contemporary invertebrates could be detected anywhere in the pipe trench sediments. A single upper molar tooth of a medium sized wild horse Equus ferus Boddaert, 1785 (13) was found close to the contact of the Pleistocene and Jurassic sediments. This is the earliest name available for the Middle and Late Pleistocene wild horse following Kaagan (2000), Whitehead (2006) and van Asperen (2009). The specimen is from a healthy young adult animal and is in fresh unabraded condition. A feature of the large mammal bones from this site is their low level of secondary calcification and absence of post-mortem weathering. In the 1975 exposure (03) remains of Wild Horse, together with Red Deer Cervus elaphus L., also occurred including fresh unabraded specimens from populations living at or near their point of burial shortly after death (14). These were concentrated at the base of a channel cut into the Charmouth Mudstone (03) overlain by reddish sands and gravels very like those observed in the 2014 pipe trench.
The work of both Kaagan (2000) and van Asperen (2009) have brought much needed uniformity to wild horse taxonomy and evolution, the absence of which hindered earlier publications (Whitehead, 1977, 1989b). The horse from the Pershore Member at Pershore, whilst robust, is less stocky than the more recent MIS 6 and MIS 2 full glacial horses from the River Avon valley; perhaps it was less well-adapted to the more extreme conditions of the Middle Pleistocene full glacial environment.
Footnote
The author has limited sealed sediment samples from a number of the units underlying February Piece and would be pleased to make some available to support U.K. scientific research by arrangement. The two lithic artefacts from this site will be donated to Evesham Almonry Museum for permanent accession.
Acknowledgements
The author wishes to thank Redrow plc and their subcontractors for facilities readily provided to undertake this study in the absence of which the evidence would have been lost forever. Dr Eline van Asperen patiently discussed some finer details of Pleistocene equid skeletal morphology.
References
Bishop, W.W., 1958. The Pleistocene geology and geomorphology of three gaps in the midland Jurassic escarpment. Philosophical Transactions of the Royal Society of London B 241:255-306.
Bowen, D.Q., 1999. On the correlation and classification of Quaternary deposits and land-sea correlation pp. 1-9 in: Bowen, D.Q. (ed.), A revised correlation of Quaternary deposits in the British Isles. Geological Society. Bath.
Bridle, A., 2012. The mid-to-late Pleistocene palaeoenvironments of the Gordano Valley, North Somerset. PhD, University of the West of England. http://eprints.uwe.ac.uk/18104/.
De Ruffignac, C., Bowen, D.Q., Coope, G.R., Keen, D.H., Lister, A.M., Maddy, D., Robinson, J.E., Sykes, G.A. & Walker, M.J.C., 1995. Late Middle Pleistocene interglacial deposits at Upper Strensham, Worcestershire, England. Journal of Quaternary Science 10:15-31.
Kaagan, L.M., 2000. The horse in late Pleistocene and Holocene Britain. Ph.D. thesis, Department of Biology, University College, London, February 2000.
Keen, D.H., 1999. The chronology of Middle Pleistocene (‘Wolstonian’) events in the English Midlands pp. 159-168 in: Andrews, P. & Banham, P. (eds), Late Cenozoic environments and hominid evolution: a tribute to Bill Bishop. Geological Society of London.
Lang, N. & Wolff, E.W., 2011. Interglacial and glacial variability from the last 800 ka in marine, ice and terrestrial archives. Climate of the Past 7:361–380.
Lisiecki, L.E. & Raymo, M.E., 2005. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. doi: 10.1029/2004PA001071.
Lowe, J.J. & Walker, M.J.C., 2014. Reconstructing Quaternary environments. Routledge, Abingdon & New York.
Maddy, D., 1999. English Midlands pp. 28-44 in: Bowen, D.Q. (ed.), A revised correlation of Quaternary deposits in the British Isles. Geological Society. Bath.
Shotton, F.W., 1968. The Pleistocene succession around Brandon, Warwickshire. Philosophical Transactions of the Royal Society of London B 254:387-400.
Shotton, F.W., 1973. English Midlands pp. 18-22 in: Mitchell, G.F., Penny, L.F., Shotton, F.W. & West, R.G., A correlation of Quaternary deposits in the British Isles. Geological Society of London Special Report 4.
Sutcliffe, A.J., 1995. Insularity of the British Isles 250000-30000 years ago: the mammalian, including human, evidence pp. 127-140 in: Preece, R.C. (ed.) Island Britain: a Quaternary perspective. Geological Society Special Publication 96.
Tomlinson, M E. 1925. The river terraces of the lower valley of the Warwickshire Avon. Quarterly Journal of the Geological Society of London81:137-169.
Van Asperen, E., 2009. Caballoid horses and late Middle Pleistocene biostratigraphy of the British Isles. Quaternaire 20:437-448.
Whitehead, P.F., 1977. Vertebrate fauna from the Carrant Main Terrace pp. 45-47 in: Shotton F.W. (ed.), The English Midlands: Guidebook for excursion A2 X INQUA Congress.
Whitehead, P.F., 1979. A tooth of Equus cf. spelaeus gallicus Prat (1968) from the Cotswolds. Quaternary Newsletter 29:18-20.
Whitehead, P.F., 1989a. Development and sequence of deposition of the Avon Valley river terraces, pp. 37-41 in: Keen D.H. (ed.), The Pleistocene of the West Midlands: Field Guide. Quaternary Research Association. Cambridge.
Whitehead, P.F., 1989b. A wild horse bone from Bredon Hill, Worcestershire. Quaternary Newsletter 59:24.
Whitehead, P.F., 1992. Terraces of the River Avon at Twyning, Gloucestershire; their stratigraphy climate and biota (with Appendices 1 & 2). Quaternary Newsletter 67:3-29.
Whitehead, P.F., 2006. Evidence of wild horse Equus ferus at South Littleton. Worcestershire Record 20:42
| 01. Avon Valley Formation. Site of Pershore Member, Allesborough Hill, Pershore, Worcestershire. |
| 03. Avon Valley Formation Pershore Member, Allesborough Hill, Pershore, Fig. 2X refers. The only colour image in existence of the April 1975 exposure. The sediments correlate with Fig. 8 of Whitehead, 1989a. The asterisk marks the position of organic channel bed fill with a temperate biota known as the Allesborough Bed. |
| 04. Avon Valley Formation Pershore Member, February Piece, Pershore. Pleistocene sands and gravels on Jurassic bedrock 5 April 2014. Intercalated pale clay seams derived from weathered exposed Jurassic sediments. Northern face of pipe trench at B in 02. |
| 05. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 5 April 2014. Pipe trench position D in 02 SO93894623 western face of north to south section. Fine bleached friable unbedded loess above gravel unit. |
| 06. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 8 April 2014. Quartzose sand unit resting on Charmouth Mudstone. North face of pipe trench close to B in 02. |
| 09. Avon Valley Formation Pershore Member, February Piece, Pershore, 9 April 2014. Diamicton filling large surface gull with a wide range of unbedded sediments and clast sizes soliflucted from upslope. Northern face of pipe trench midway between A and B in 02. Trowel is 28 cms long. |
| 10a Avon Valley Formation Pershore Member, February Piece, Pershore, 8 April 2014. Close up of ferrocreted sand and gravel northern face of pipe trench close to A in 02. |
| 10b. Avon Valley Formation Pershore Member, February Piece, Pershore, 9 April 2014. Shows dominantly sub-angular sand grains as in 10a at higher magnification. © P.F. Whitehead |
| 11a. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 8 April 2014. Northern face of pipe trench (08) close to A in 02 SO93744620. Thick discoidal scraper of Middle Palaeolithic affinity worked on poor quality grey cherty flint of easterly origin. © P.F. Whitehead. |
| 11b. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 8 April 2014. Reverse of 11a. This surface is defined exclusively by centripetal flake scars. 11c shows the heavily utilised scraping edge at the top edge of the artefact in the larger images. © P.F. Whitehead. |
| 11c Avon Valley Formation Pershore Member, February Piece, Pershore, 8 April 2014. The heavily utilised scraping edge at the top edge of the artefact in the larger images in 11a and 11b. © P.F. Whitehead |
| 12a. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 9 April 2014. Palaeolithic concave scraper on fine-grained micaceous Jurassic siltstone the scraping face showing numerous unidirectional utilisation and/or pressure retouch removals. © P.F. Whitehead. |
| 12b. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 9 April 2014. As 12a showing the concave scraping face defined by numerous unidirectional utilisation and/or pressure retouch removals. © P.F. Whitehead. |
| 13. Avon Valley Formation Pershore Member, February Piece, Pershore, Worcestershire, 3 April 2014. Left upper tooth of wild horse Equus ferus Boddaert, 1785, probably fourth premolar. Base of coarse sands and gravels near 2C in 02 SO93864622. © P.F. Whitehead. |
| 14. Wild Horse Equus ferus Boddaert, 1785. Mandibular symphysis with retained incisor teeth of animal about 11 years old. Avon Valley Formation Allesborough Bed, Allesborough Hill, Pershore, Worcestershire, April 1975. It should be noted that this specimen was broken shortly after death probably through human butchery techniques to extract fatty marrow from the rami. © P.F. Whitehead. |
Worcestershire Record | 37 (November 2014) page: 36-45 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 29-30 | Worcestershire Biological Records Centre & Worcestershire Recorders
Climbing Corydalis Ceratocapnos claviculata (L.) Lidén (Ranunculales, Papaveraceae) flowering on the Malvern Hills during December
P.F. Whitehead
Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH Email: paul@thewhiteheads.eu
Climbing Corydalis Ceratocapnos claviculata (L.) Lidén is a well-known inhabitant of the Malvern Hills and is generally regarded as an annual (e.g. Maskew, 2014). On 31 December 2014 near the vice-county boundary at Hangman’s Wood, Malvern Hills (VC37 SO73 251m a.s.l.) I observed a number of well-established plants of C. claviculata in flower which in my experience was novel.
In their seminal work on this plant Voss et al. (2012) accepted that in some situations C. claviculata could be a ‘short-term perennial’ and it may be that this explains the situation in this case. This is achieved ‘in some sheltered locations’ by winter survival. In Papaveraceae the same phenomenon can sometimes be observed in England in Eschscholzia in which individual plants may perennate once in suitable climatic conditions.
However during 2014 I was able to demonstrate that some plants usually regarded as annuals were able to complete two complete generations in one calendar year. Whatever the explanation for this phenomenon on the Malvern Hills it represents atypical phenology probably due mostly to climatic factors. Given that the flowers of C. claviculata are partially self-pollinated the production of seeds from this unseasonal flowering is entirely possible.
References
Maskew, R. 2014. The Flora of Worcestershire. 810pp. Published privately.
Voss, N., Welk, E., Durka, W. & Eckstein, R.L. 2012. Biological flora of Central Europe: Ceratocapnos claviculata (L.) Lidén. Perspectives in Plant Ecology, Evolution and Systematics 14: 61-77.

Fig. 1. Climbing Corydalis Ceratocapnos claviculata (L.) Lidén in flower near the vice-county boundary at Hangman’s Wood, Malvern Hills, 31 December 2014.
Worcestershire Record | 37 (November 2014) page: 29-30 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 19-20 | Worcestershire Biological Records Centre & Worcestershire Recorders
Gonia picea (Robineau-Desvoidy, 1830) (Diptera, Tachinidae) and other significant invertebrates from the Malvern Hills (VC37 SO74)
P.F. Whitehead
Moor Leys, Little Comberton, Pershore, Worcestershire, WR10 3EH. Email: paul@thewhiteheads.eu
Introduction
The historical development of the entomofauna of the Malvern Hills is imperfectly understood but there seems little doubt that, when further data has been assembled, its terrestrial fauna will rank amongst the more significant in Britain. The hills are known not only for their distinguished relict species (Whitehead 1989, 1996, 2007, 2010), but also for their ability to collect and assemble convected and windblown insects. This is a well-known phenomenon in mountainous regions but is especially noted at particular times on the Malvern Hills (Whitehead, 1994) which on the east side rise abruptly to mountain altitude above the Jurassic plain. This note documents selected observations made on 3rd May 2014 in full sun in rather cool windy conditions, when the ladybirds Harmonia axyridis (Pallas, 1773) and Coccinella septempunctata (L.) and the coprophilous scarab beetle Onthophagus similis (Scriba, 1790) were observed on the summit of North Hill. Six species of elaterid beetle were encountered without particular searching and a few Painted Lady and Red Admiral butterflies were moving north (03) along the high crests. The former were notable sun-bleached indicating an origin well to the south.
Selected observations 3 May 2014
Liogluta microptera Thomson, 1867 (Coleoptera, Staphylinidae).
This genus of staphylinid beetles is what may be termed ‘difficult’ due to intraspecific variation which extends also to the genitalia. Old records should be interpreted with care and I am grateful to Marc Tronquet for discussing various aspects of this genus. A female L. microptera from around the Happy Valley spring (264 m O.D.) would seem to be the only acceptable Worcestershire record to date.
Gonia picea (Robineau-Desvoidy, 1830) (Diptera, Tachinidae)
A single worn example of this scarce tachinid fly was observed at the summit of North Hill (398 m O.D.) for about an hour, typically flying short distances before resettling in grass (01). Various muscid flies were observed in full exposure at the same location. Pollen on the fly suggests that it had been visiting nearby flowers of Bilberry Vaccinium myrtillus L. Gonia picea is predominantly a parasitoid of noctuid moths and a previous Worcestershire record was provided by Bingham (2012). In this case however the montane habitat would seem to be unique in Britain although Belshaw (1993) makes reference to the attraction of hilltops for some male tachinids.
Geophilus easoni Arthur, Foddai, Kettle, Lewis, Luczynski, & Minelli, 2001. North Hill, Malvern Hills, 3 May 2014
An example of this species, probably a male (02), was observed on North Hill (397 m O.D.). Until recently this terrestrial species was confused with Geophilus carpophagus Leach, 1814, a quite different arboreal species which also occurs on the Malvern Hills. Geophilus easoni is likely to be under-recorded in Worcestershire from where there appear to be no published records.
Pictures: extended captions
01. Worn Gonia picea (Robineau-Desvoidy, 1830), North Hill, Malvern, 3 May 2014. © P.F. Whitehead.
02. Geophilus easoni Arthur, Foddai, Kettle, Lewis, Luczynski, & Minelli, 2001. North Hill, Malvern Hills, 3 May 2014. © P.F. Whitehead.
03. A rather faded probably far-travelled Vanessa atalanta (L., 1758) (Lepidoptera, Nymphalidae) resting at Worcestershire Beacon, Malvern Hills (398 m O.D.), 3 May 2014. © P.F. Whitehead.
Discussion
The time-honoured entomofauna of the Malvern Hills includes many species which are negatively anthrophilic; a fauna of ‘wild places’. With the increasing development of human settlements negatively and positively anthrophilic faunas will become, and are becoming, increasingly polarised in many parts of Britain and Europe. The Malvern Hills entomofauna include many such ‘wild’ species that shun human activity. Notable amongst these is Onthophagus similis for which the Malvern Hills form a regional population focus. This group of dung beetles declined rapidly following broad climatic deteriorations of the past few thousand years and again more recently in the light of changing animal husbandry practices.
All of this will pose something of an increasing challenge in terms of land management and human access; recent developments regarding pastoralism will undoubtedly have brought benefits to the more ancient elements of the upland entomofauna.
References
Arthur, W., Foddai, D., Kettle, C., Lewis, J. G. E., Luczynski, M. & Minelli, A. 2001. Analysis of segment number and enzyme variation in a centipede reveals a cryptic species Geophilus easoni sp. nov. and raises questions about speciation. Biological Journal of the Linnaean Society 74:489-499.
Belshaw, R., 1993. Tachinid flies. Handbooks for the identification of British insects 10:4a(i). Royal Entomological Society of London.
Bingham, J., 2012. Gonia picea (Robineau-Desvoidy, 1830) Tachinidae, Diptera and Melanimon tibialis (Fabricius, 1781) Tenebrioninae, Coleoptera, Recorded on the Devil’s Spittleful Nature Reserve 2012. Worcestershire Record 32:14.
Whitehead, P.F., 1989. An inland record of Calathus mollis (Msh.) from Worcestershire. Entomologist’s Monthly Magazine 125:198. [Note: in reality this refers to Calathus cinctus Motschulsky which was not formally recognised as British until after this paper was published].
Whitehead, P.F., 1994. The role of meteorology in an unusually mixed assemblage of Coleoptera. Entomologist’s Monthly Magazine 130:200.
Whitehead, P.F., 1996. A modern British record of Aleochara maculata Brisout 1863 (Coleoptera, Staphylinidae) with reference to its ecology. Entomologist’s Gazette 47:253-254.
Whitehead, P.F., 2007. New autecological data for Enoicyla pusilla (Burmesiter, 1839) (Trichoptera:Limnephilidae) from the Worcestershire Malvern Hills. Entomologist’s Gazette 58:26-28.
Whitehead, P.F., 2010. An overlooked Worcestershire insect, the Snow Flea. Worcestershire Record 28:15.

01. Worn Gonia picea Malvern, 3 May 2014. P.F. Whitehead

02. Geophilus easoni, Malvern Hills, 3 May 2014. P.F. Whitehead.
![]()
03. Vanessa atalanta Worcestershire Beacon. P.F. Whitehead
Worcestershire Record | 37 (November 2014) page: 19-20 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 8 | Worcestershire Biological Records Centre & Worcestershire Recorders
Gymnomerus laevipes (Shuckard, 1837) (Hymenoptera, Vespidae) new to Worcestershire and an amendment to the published record
P.F. Whitehead.
Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH Email: paul@thewhiteheads.eu
Reference was recently made (Whitehead, 2013) to the finding of Ancistrocerus oviventris (Wesmael, 1836) at Grafton Wood, Worcestershire on 25 August 2013. I recently reviewed the specimen and decided to send it to Dr Michael Archer for examination; he found that it was a female Gymnomerus laevipes (Shuckard, 1837). This published record of A. oviventris is therefore untenable.
Richards (1980) keys the subfamily Eumeninae and states that the head of Gymnomerus has ‘two approximate occasionally fused pubescent pits’ and illustrates them as figure 25. In the present example these pits are very small, much smaller than this figure 25 and situated well behind the eyes and might be passed over; this and a subsequent failure to attend to other points of detail lead to the specimen being misidentified. Dr Archer also stated that when considering the identity of certain individuals reliance is sometimes placed on one or a few characters which require to be visible and clearly evident.
The review of this specimen by Dr Archer, to whom I grateful, is fortuitous because Trevis (2004) cited no records of G. laevipes in Worcestershire between 1990 and 2004, although it is understood that a 2008 record from Worcestershire will be cited shortly (Trevis, in press). The Grafton Wood specimen therefore helps to infill a further gap along the northern range edge. Gymnomerus laevipes is believed to subsist on larvae of weevils of the genus Hypera. Although it is recorded here in the woodland context and some species of Hypera will overwinter under the bark of standing trees, they are dominantly grassland weevils so that G. laevipes will be utilising the landscape mosaic of clearings, fields and woodland ecotones.
References
Trevis, G. 2004. Aculeate Hymenoptera in Worcestershire. Worcestershire Record 16:27-28.
Trevis, G. in press. A review of Worcestershire aculeate Hymenoptera with species notes.
Whitehead, P.F., 2014. Notable invertebrates including Rhizophagus oblongicollis Blatch & Horner, 1892 from the Grafton Wood area of Worcestershire during 2013. Worcestershire Record 35:47-48.
Worcestershire Record | 37 (November 2014) page: 8 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 46-47 | Worcestershire Biological Records Centre & Worcestershire Recorders
Taxonomy, clavicipitaceous fungi and the juggler of molecules
P.F. Whitehead.
Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH Email: paul@thewhiteheads.eu
Introduction
For many of us concerned with the systematic position of organisms their phyletic placement within recognised taxa are the hooks on which the relationships of life forms are hung. In recent times the application of mitochondrial DNA analyses has begun to revolutionise our understanding of these relationships. To some extent thereby hangs a problem, or more correctly, problems, because the light provided by molecular laboratory studies often casts shadows over our wider perception.
The biological assessor, the student of faunistics, or the writer of identification handbooks will always be hindered if species can only be recognised precisely by sophisticated laboratory techniques. The problem might be contrasted in this way: while biologists race to describe unknown life forms faced with imminent extinction molecular scientists construct or deconstruct clades and determine phyletic exactness in the wider context of all life. Which, might one argue if the question is not unduly specious, is the more relevant? An illustration using fungi may or may not help to provide an answer.
Nomenclature, taxonomy and entomogenous fungi
Taxonomically valid species require a scientific published description, a valid binomial and an available type specimen designated to support the description. This may be a holotype, a subsequently designated lectotype together with an accompanying series of paratypes. Usually, but not always, a species is the product of embryonic fertilisation. Exceptions to this include parthenogenesis e.g. in some weevils where an imago is the result of an unfertilised embryo, and amongst plants where the same process creates rafts of ‘microspecies’ through apomixis. Valid binomials have traditionally been applied to all of these variations.
01. Cordyceps sp. (? militaris (L., Fr.) Fr., 1818) (Hypocreales:Cordycipitaceae) on noctuid moth pupa Pwll Mawr Wood, Wye Valley VC35 SO51 135 m a.s.l. 13 August 2013 © P.F. Whitehead.
Nomenclatural issues from the Kingdom of fungi have perplexed mycologists (and me!) for decades. Historically, Article 59 of the International Code of Botanical Nomenclature permitted mycologists to give asexually reproducing fungi binomials distinct from those applied to their sexual states; this practice was discontinued only as recently as 2013. Many fungi from a range of taxonomic orders reproduce both sexually and asexually. When mycologists applied binomials to asexual forms, which they termed anamorphs, they were often unaware of the sexual states of these species, which they termed teleomorphs, or as we shall see, even where they existed. The 2013 ruling meant that one fungus cannot bear two names and that the name of the sexual stage should have precedence.
For one group of fungi, the entomogenous ascomycotine Hypocreales, this will inevitably result in a significant revision of established texts. To some extent this process has already begun (Sung et al., 2007). A key entomogenous fungal genus is Cordyceps Fries which produces as its sexual stage characteristic elongate often clavate stromata (01) eventually with fertile expanded heads (Samson, Evans and Latgé, 1988). A species of special regional importance is Harposporium bredonense Evans & Whitehead, 2005 known globally only from Bredon Hill and Tiddesley Wood, Pershore, where its host in both cases is arboreal cerambycid beetles.
A significant problem of applying binomials to anamorphs of the more than 400 species of Cordyceps can be understood when it is recognised that they include at least ten genera thereby making for a large number of potentially ‘synthetic’ relationships. What this boils down to is that taxonomy is presently waiting on the results of informed molecular studies but more especially that field workers are waiting on how molecular scientists might translate their work into helpful results. Sung et al. (2007) succeeded in this regard by indicating that the structure, colour and texture of the stromata are highly significant in establishing phylogenies in some entomogenous fungi.
02. Cordyceps bassiana Li, Li, Huang & Fan, 2001 in its anamorphic state ‘Beauveria bassiana (Bals.-Criv.) Vuill.’ (Hypocreales:Cordycipitaceae) on Nebria brevicollis (F., 1792) (Coleoptera:Carabidae) Stanway woods complex, Gloucestershire VC33 SP03 184 m a.s.l. 15 November 2014. Further molecular studies are likely to require that this determination be revised. © P.F. Whitehead.
For the moment the fungus illustrated here in 01 can only be determined to Cordyceps sp. It may well be the same species as the anamorphic Paecilomyces farinosus (Holmsk.) Fr. which is now most usually placed in Isaria! This is the species that Pacioni and Frizzi (1978) demonstrated was the anamorph of Cordyceps memorabilis (Ces.) Sacc., 1879. However, whilst 01 shows well-developed stromata there appear to be no fertile coloured stromatic heads; on this basis it could belong to Cordyceps militaris (L., Fr.) Fr., 1818 and this may well be its true identity. As molecular studies progress images such as these are likely to become easier to assign.
As a final confirmation of the revolutionary findings of molecular systematics in this group one may cite the well-known entomogenous fungus Beauveria bassiana (Bals.-Criv.) Vuill. (02). Ignoring for the moment whether or not this is a species or a species-complex it is presently held to be the anamorph of Cordyceps bassiana Li, Li, Huang & Fan, 2001, the teleomorphic stage which is apparently up to now only known from the East Palaearctic Region.
Conclusion
Continuing molecular research on this most challenging group of organisms will hopefully bring further clarity and resolution of taxonomic difficulties, especially given the enormous geographical ranges of some species. If published findings can be applied to field work my question (vide supra) will to some extent have been answered.
References
Pacioni, G. & Frizzi, G. 1978. Paecilomyces farinosus the conidial state of Cordyceps memorabilis. Canadian Journal of Botany 56:391-394.
Samson, R.A., Evans, H.C. & Latgé, J.-P. 1988. Atlas of entomopathogenic fungi. 187 pp. Springer-Verlag.
Sung, G.-H., Hywel-Jones, N-L., Sung, J.-M., Luangsa-ard, J.J., Shrestha, B. & Spatafora, J.W. 2007. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in mycology 57:5-59.
Whitehead, P.F. & Evans, H.C. 2005. Entomogenous fungi of arboreal Coleoptera from Worcestershire England including the new species Harposporium bredonense. Mycological Progress 4:91-99.
Whitehead, P.F. 2008. A second record of the entomogenous fungus Harposporium bredonense Evans & Whitehead, 2005 (Clavicipitales). Entomologist’s Monthly Magazine 144:162.

01. Cordyceps sp on noctuid moth pupa. Paul Whitehead.

02. Cordyceps bassiana on Nebria brevicollis. Paul Whitehead.
Worcestershire Record | 37 (November 2014) page: 46-47 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 11-12 | Worcestershire Biological Records Centre & Worcestershire Recorders
The Mordellidae or ‘tumbling flower beetles’
Paul Whitehead
The Mordellidae or ‘tumbling flower beetles’ has always challenged human comprehension. The late Mr A. A. Allen, one of the greatest of the recent British coleopterists, described new species of Mordellistena in both 1995 and 1999 neither of which have stood up to subsequent scrutiny. The group is generally well known for the apical abdominal segment being extended to form a so-called pygidium (Gr. pygidion = rump). This is especially conspicuous in the black Mordellistena which are especially speciose in southern Europe and which mostly breed in the rigid stems and rootstocks of Asteraceae, including Artemisia spp. and occasionally those of other groups such as Campanulaceae (e.g. Jasione).
Only three of the 12 British species of Mordellistena are not black viz. the scarce widespread M. neuwaldeggiana (Panzer, 1796) which is more or less uniformly brownish-orange and thus immediately recognisable; M. humeralis (L., 1758) which is variegated black and dull yellow-orange rarely with the pronotum darkened and M. variegata (F., 1798) which (in numerous examples seen) has clear orange vittae running obliquely away from the elytral humeri and the pronotum usually darkened but paler laterally and with the antennae longer. These three species are arboreal as larvae in a wide range of deciduous trees usually in soft delignified wood; M. neuwaldeggiana occurs in traditional orchards. In the English midlands I have numerous records of these species with the exception of M. humeralis which I have not yet seen in Britain. Male M. variegata have diagnostic subcircular last palpal segments whereas these are not dilated in male M. humeralis which also has relatively shorter antennae.
Other genera likely to be encountered in the region are Mordellochroa and Variimorda. Female Mordellochroa abdominalis (F., 1775) are unique and unmistakeable in the British fauna being black with clear red pronota; the males have darker pronota and may be confused with species of Mordellistena and Mordella. Ash (Fraxinus excelsior L.) is a known larval host in Worcestershire. Variimorda villosa (Schrank, 1781) is the only British representative of the genus and may be recognised by the pattern of shimmering bronze hairs forming transverse fasciae on the black elytra. Both of these robust species are arboreal as larvae although V. villosa has a demonstrable preference for Salicaceae in riparian situations. Mordella, a genus of mostly robust black species includes two British species which are either rare or localised; M. holomelaena Apfelbeck, 1914 may occur in the region but I am not aware of modern records.
For anyone wishing to acquaint themselves with the finer details of this group I would recommend perusal of Batten, R., 1986. A review of the British Mordellidae (Coleoptera). Entomologist’s Gazette 37:225-235. They might also consider forming a collection taking no more than a minimal number of specimens. As ‘tumbling flower beetles’ mordellids prefer to visit flowers with exposed nectaries, notably those in Rosaceae and Apiaceae and with records also from flowers of Sycamore Acer pseudoplatanus L. Finally (with a few clear exceptions) do not rely on the internet to inform decisions; many such images are based on misidentifications.
Worcestershire Record | 37 (November 2014) page: 11-12 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 9 | Worcestershire Biological Records Centre & Worcestershire Recorders
Two genera of carabid beetles overwintering in a single air cell at Birlingham, Worcestershire
P.F. Whitehead.
Moor Leys, Little Comberton, Pershore, Worcestershire WR10 3EH Email: paul@thewhiteheads.eu
The overwintering by beetles of various families in terrestrial or arboreal air cells is reasonably well-known (Whitehead, 1992) especially in riparian situations. Amongst the genus Carabus in which it is frequently encountered, Carabus granulatus L., 1758 is noted for its ability to use soft rafted wood or suitable standing trees in which to cut and maintain air cells. The cells are usually somewhat larger than a single beetle and reinforced with a barrier of compressed chewed wood which creates a cell that helps resist the ingress of rising flood water and insulate the insects.
In two contributions the author has demonstrated that on occasion pairs of the same species may co-operate to construct and maintain a single cell. At Bialowieza National Park, Poland, on 27 September 1998 a pair of Carabus cancellatus Illiger, 1798 were found in an established air cell under the appressed bark of a long-fallen Norway Spruce Picea abies L. thus raising interesting questions regarding pair-bonding, division of labour, survival and reproductive success (Whitehead, 2001). During January 2006 at Slimbridge, Gloucestershire, three individual Carabus granulatus had co-operated to cut a single overwintering cell and were found together in it (Whitehead, 2006). The cell had a maximum depth of 31mm and a maximum breadth of 36mm and was situated beneath the trunk bark of a fallen Crack Willow Salix fragilis L. on the grazing levels of the estuarine River Severn.
A finding at Birlingham, Worcestershire (SO94 12 m a.s.l.) on 26 December 2014 is perhaps unique, for it involves the cooperation of two individuals of different genera of carabid beetles, namely Carabus granulatus and Pterostichus niger (Schaller, 1783), in creating and maintaining a single large air cell defined circumferentially by macerated wood fragments deep inside a soft flood-rafted willow trunk Salix sp. Although it remains unknown how the two insects cooperated in this case it seems that both recognised the mutually conferred benefits of their labours and were content to cohabit in this way.
References
Whitehead, P. F. 1992. The floodplain Coleoptera of the River Avon, Worcestershire, England, with provisional diagnoses of ancient assemblages. Elytron 6:15-33.
Whitehead, P. F. 2001. Pair-bonding in Carabus cancellatus Ill. (Col., Carabidae). Entomologist’s monthly Magazine 137:114.
Whitehead, P. F. 2006. Evidence of social organisation in overwintering Carabus granulatus L., 1758 (Col., Carabidae). Entomologist’s monthly Magazine 142:141.
Worcestershire Record | 37 (November 2014) page: 9 | Worcestershire Biological Records Centre & Worcestershire Recorders
Worcestershire Record | 37 (November 2014) page: 54-66 | Worcestershire Biological Records Centre & Worcestershire Recorders
A multi-scalar analysis of habitat characteristics associated with the Noble Chafer beetle Gnorimus nobilis in south Worcestershire
Fred Windsor
Introduction
Anthropogenic activity and intensification of agricultural production have facilitated a decline in species diversity across Europe throughout the 21st century (Pimm et al., 1995). A key driver of this change was the post-WWII transition from agricultural subsistence to intensive mass production instigating dramatic shifts in the character of agricultural landscapes (Bignal and McCracken, 1996; Robinson and Sutherland, 2002; Stoate et al., 2009). Contemporary management focuses on achieving the maximum profit per hectare (Rounsevell et al., 2003), which in many cases promoted the adoption of alternative forms of more profitable agriculture (Paracchini et al., 2007; Sharples, 2007; Barker et al., 2011). Orchard habitats within the UK saw a paradigm shift away from traditional management (Koziell, 2000; Robertson and Wedge, 2008), concentrating activity in relatively small pockets of land, thousands of hectares smaller than previously (Barker et al., 2011). Furthermore, altered management facilitated an increased utilisation of pesticides, herbicides and fungicides which are directly deleterious to wildlife (Epstein et al., 2001; Barker et al., 2011). Although traditional management still persists within orchard agriculture (Cloke and Jones, 2001; Duarte et al., 2008), a loss of favourability continues to reduce its utilisation (Tscharntke et al., 2005). This is detrimental for biological diversity as traditionally managed agricultural and arboreal habitats are extremely species rich (Berg et al., 1994; Bignal and McCracken, 1996; Alexander, 1999; Ranius, 2001; Plieninger et al., 2006; Orłowski and Nowak, 2007; Müller et al., 2008; Bailey et al., 2010).
The transition away from traditional, low intensity management has critically affected the volume of decay habitats, which are declining throughout Europe (Grove, 2002a; Ranius and Fahrig, 2006; Ranius, 2007). Decreasing decay has negatively impacted many organisms, especially those within the saproxylic habit (Nilsson, 1997; Ranius and Wilander, 2000; Brown and Schmitt, 2001; Löfman and Kouki, 2001; Benton et al., 2002; Jonsell and Nordlander, 2002; Similä et al., 2003; Philpott et al., 2008; Ranius and Roberge, 2011). Saproxylic species are defined as organisms which possess a distinct association with either standing or moribund decaying wood and/or the products of such decay (Speight, 1989; Alexander, 2008a). The saproxylic habit encompasses entire families (Webb et al., 2008) and occupies a distinct range of habitats (Grove et al., 2002a). Thus it appears imperative that undisturbed sites of old-growth orchards are conserved in order to maintain saproxylic community integrity (Niemelä, 1997).
Beetles (Coleoptera) are an especially abundant and diverse order, which often significantly contribute to the aforementioned diversity of agricultural habitats (Bouchard et al., 2009; Groombridge, 1992). The Noble Chafer beetle Gnorimus nobilis is a visually conspicuous saproxylic species (Krikken, 1984: Whitehead, 2003; PTES, 2008a), endemic to large areas of Europe. Its dominant range spans the north-west of the continent with less frequent distributions extending southwards (Osborne, 1974; Tauzin, 2000). Within the UK the anthropogenic influences have altered the structure and composition of arboreal habitats (Wilson, 2002; Hopkins and Kirby, 2007), in turn fragmenting decay habitat (Hannah et al., 1995). This has significantly altered the distribution of G. nobilis (Alexander, 2008b). Contemporary populations exist within central to southern England and although covering a relatively broad geographical area, limited dispersal restricts G. nobilis to a finite number of waning habitats (Cooter et al., 1991; Alexander, 2008b). Subsequently G. nobilis, as well as other saproxylic species, is vulnerable and at risk of extinction (Smith, 2003; Evans and Whitehead, 2005; JNCC, 2007; Macdonald et al., 2007; Alexander, 2008b; Nieto and Alexander, 2010; JNCC, 2010; Sebek et al., 2013).
Regions associated with G. nobilis are concomitant with traditionally managed orchard horticulture (Robertson and Wedge, 2008), which appears to be the dominant habitat of G. nobilis within the UK (Alexander, 2002; Whitehead, 2003; Alexander, 2008b; PTES, 2008a; Lush et al., 2009; Alexander and Bower, 2011). Nevertheless, alternate, non-horticultural populations have been recorded (Alexander, 2010). Traditional orchard habitat is defined as: “… [sites] where at least 5 fruit trees must be present with no more than 20m between their crown edges.” (Burrough and Robertson, 2008, pp. 27). Management is vital (Kirby et al., 1995). This encompasses three strategies: (1) The generation of low canopy densities (Robertson and Wedge, 2008); (2) the complete absence of artificial pesticides and fertilisers (Herzog, 1998; Cordrey et al., 2008); and (3) periodic mowing or grazing (Burrough and Robertson, 2008). This in turn allows for a large quantity of decay habitat (Whitehead, 2003; Schroeder et al., 2007), suitable for saproxylic species.
The association with orchard agriculture, however, only occurred contemporaneously. Osborne (1974) showed that during the early Flandrian period G. nobilis populations were associated with pioneer deciduous woodland. Therefore a transition from deciduous woodland to orchard habitat has transpired. This is most likely due to anthropogenic disturbance within deciduous woodland reducing decay quantity, and therefore suitability.
In spite of globally diminishing decay habitats, the UK maintains the largest reserves of old-growth habitat in northwest Europe (Alexander, 1998). Nevertheless, the status of such habitat remains in the balance, with increasing orchard vulnerability (Marshall, 2008; Widdicome et al., 2008) coinciding with losses of old-growth habitat diversity (Sippola et al., 2002; Grove, 2002d). Therefore further research is vital for supporting existing conservation whilst further increasing the preservation of old-growth habitat (Whitehead, 1992; 1997).
This exploratory study is concerned with the unique habitat characteristics produced by traditional orchards with regards to G. nobilis presence throughout the landscape of south Worcestershire. Within this assessment a previously unexplored scale of inhabitance is examined, allowing for further ecological knowledge regarding G. nobilis to be acquired. This provides a novel contribution to a still relatively under-researched field of British entomology. The implications of this research are particularly important for the continuation of traditional management within the landscape, which in turn appears to facilitate the persistence of this species within the UK.
Scientific Background
Literature regarding G. nobilis is sparse, with relatively few studies explicitly analysing the characteristics of occupied orchards. Therefore, the analysis of research regarding similar saproxylic beetle species provides direction within this study. Habitat characteristics of inhabited sites are of particular interest due to the unexplained transition from deciduous woodland and the explicit absence of G. nobilis from intensively managed sites (Alexander, 2008b).
Scale is important to consider when assessing habitat biodiversity (Nilsson et al., 2001; Murphy and Lovett-Doust, 2004; Bergman et al., 2012). Environmental characteristics transcending multiple spatial scales have been observed affecting the distribution of organisms (Elith and Leathwick, 2009) and specifically arthropods (Jonsell et al., 1998; Meggs et al., 2003; Holland et al., 2004; Cardoso et al., 2009). These characteristics can be segregated based on the spatial scale over which they operate (Holland et al., 2005) as well their influence on species distribution (Mackey and Lindenmayer, 2001; Chase, 2007; Lindo and Winchester, 2009). Within the literature two distinct categories are observed: Habitat/site and landscape. Within these broad categories several discrete sub-classifications are observed.
Therefore it can be seen that a variety of spatial scales need to be analysed so as to comprehensively detail the population distribution of saproxylic beetles (Bergman et al., 2012). Complexity is provided by the holometabolous nature of G. nobilis, which exhibits several instars throughout development (Whiting, 2002; Whitehead, 2003). The developmental stage of an organism alters how environments and resources are perceived (Murphy and Lovett-Doust, 2004). Due to this an appreciation of the requirements of G. nobilis throughout its multiple developmental stages will be presented throughout this dissertation.
Landscape Scale
Landscape characteristics
The landscape surrounding traditional orchards has received little attention, with regards to land-use. However, land-use may be significant as G. nobilis adults have been observed nectaring on Apiaceae (Whitehead, 2003), and may rely on open landscapes, devoid of obstacles in order for successful dispersal. Not only is the contemporary land-use important, the longevity of landscape features appears significant for G. nobilis (Alexander, 2008b), with the temporal prerequisites of decay production limiting saproxylic species to well-developed habitats (Cardosa et al., 2009).
Landscape characteristics create unique interactions, transcending multiple scales (Stoner and Joern, 2004) which may introduce complexities, some of which are not yet fully understood. Thus through an analysis of multiple scales, an in depth picture of interactions can be constructed. An assessment of structural and functional connectivity (Collinge, 2000; With et al., 1999) will elucidate the relative importance of the landscape; potentially highlighting the downward influences impacting the suitability of local habitat within the wider landscape matrix (Sirami et al., 2008). Such multi-scalar interactions have not been assessed for G. nobilis but have significantly influenced other arthropod species (Fetridge et al., 2008).
Habitat/Site scale
Orchard characteristics
G. nobilis is explicitly absent from intensively managed orchard stands (Whitehead, 2003; Alexander, 2008b), thus specific influences at this scale may provide an insight into significant habitat characteristics. The nature of orchard sites modifies local habitat climate, e.g. temperature and wind speed; thereby influencing species presence (Jeanneret et al., 2003; Frank et al., 2009; Chiari et al., 2012; Lachat et al., 2012). It appears high density cultivation within intensive orchards makes them unsuitable for G. nobilis through these mechanisms (Atkinson and Winnall, 2008). High density canopies create shade and prevent adequate sunlight penetration (Franklin et al., 2002). Thus, cool, damp microclimates are produced, providing conditions that are highly unfavourable for saproxylic species dependent on radiative heating for incubation (Alexander, 2008b) and the production of decay products, on which larvae feed (Zdenĕk et al., 2012). These effects are exacerbated for G. nobilis as it is a particularly temperature sensitive species (Renault et al., 2005). However, as of yet an examination of relative density within traditional orchards is absent. Previous research regarding G. nobilis has be predominantly qualitative and observational, thus quantification of relationships may provide more substantial evidence. Habitat volume, density and heterogeneity significantly influence the colonisation of habitat (Murcia, 1995; Similä et al., 2006), with important effects including edge-area ratios (Ewers et al., 2007) and inter-habitat connectivity (With and Crist, 1995). The assessment of multiple orchard factors within this study looks to encapsulate some of these effects in a quantifiable manner.
Tree characteristics
Progressing vertically down through the spatial hierarchy, we reach individual tree characteristics. The production and availability of local habitat, within the wider context is vital for determining the distribution of species (Martikainen, 2001). This is especially significant as the high diversity associated with arboreal habitats is generally created by site specific conditions (Ranius, 2006). These characteristics, however, are not always observed providing a significant influence for saproxylic species (Similä et al., 2002). Although, in the case of G. nobilis local habitat influences appear dominant (Lush et al., 2011; Alexander 2008b; Hurt and Burrough, 2009). Many characteristics at this scale have been assessed, although conclusions are generally inconsistent; with studies assessing both tree species and girth tending to contradict one another, with few regular trends emerging (e.g. Alexander, 2008b; Lush et al., 2009; Alexander and Bower, 2011).
G. nobilis and other saproxylic species are particularly reliant on the suitability of decay habitat (Grove, 2002c; Alexander, 2003; Jacobs et al., 2007; Lush et al., 2009; Ranius et al., 2009a; Alexander and Bower, 2011). Yet previous research has only focused on G. nobilis populations with regards to the presence or absence of this resource. Results, however, indicated that the absence of G. nobilis from intensively managed orchards may be a product of low heartwood and decay accumulation (Atkinson and Winnall, 2008; Lang, 2001; Robertson and Wedge, 2008).
A further defining feature of previous research is that only inhabited trees have been assessed with little investigation of the differences between colonised and un-colonised habitat. Comparisons between these habitats may be particularly successful in the case of G. nobilis as highly specialised saproxylic species may be distributed across relatively few suitable host trees (Davies et al., 2008). Therefore characteristics of inhabited sites may be concise in comparison to unsuitable features within uninhabited sites.
Microhabitat characteristics
Microhabitat characteristics refer to the conditions found within individual bole features as defined by Michel and Winter (2009) and Read (2000). They are vital in maintaining the diversity and distribution of species within arboreal ecosystems (Rotheray, 2013; Larrieu et al., 2012) and have been shown to influence the distribution and abundance of saproxylic species across habitats (Tahvanainen, 1972; Jonsson et al., 2005; Wermelinger et al., 2007). Such variations enable greater local variation than larger scales (Jonsson et al., 2005). Although, some characteristics provided by vegetative micro-features are less dynamic and can stabilise microclimatic variability (Rukke and Midtgaard, 1998; Molina-Montenegro et al., 2009). No work has been completed on microhabitat characteristics with regards to G. nobilis, yet as seen, similar species are influenced at this scale. Research suggesting weak or redundant relationships between micro-scale characteristics, diversity and abundance of saproxylic species (e.g. Økland et al., 1996; Siitonen and Saaristo, 2000), are generally derived from high dispersal strength of the species (Økland et al., 1996). In the case of G. nobilis observational research suggests that it may be a poor disperser (Whitehead, 2003), and thus local features may dominate.
Research questions
Considering previous research on G. nobilis and comparisons with other similar species several themes are highlighted. The overarching question considered by this dissertation is whether G. nobilis displays associations with specific habitat characteristics throughout the south of Worcestershire. This encompasses analysis of four distinct scales: 1) Landscape; 2) Orchard; 3) Tree; and 4) Microhabitat.
Across these four scales there are several research objectives:
Assess the differences between inhabited and uninhabited orchard characteristics within south Worcestershire.
Assess the relative influence of each distinct spatial scale on the distribution of G. nobilis within south Worcestershire.
Assess the relative influence of individual habitat characteristics on the distribution of G. nobilis within south Worcestershire.
Methodology
Study area
South Worcestershire has long been recognised as a stronghold for horticulture (Robinson, 1983), providing suitable habitat for G. nobilis populations; confirmed by records of G. nobilis dating back to 1945 (Evans and Whitehead, 2005). Historical records of G. nobilis indicators and sightings within Worcestershire are prevalent throughout south Worcestershire, maintaining a particularly large density of contemporary records.
The sites analysed within this dissertation are dispersed across central-south Worcestershire, encompassing approximately 25km2. Sites were provisionally selected using the Natural England traditional orchard habitat survey, which was part of a wider habitat cataloguing scheme instigated by Natural England in 2011 (Natural England, 2012). Remotely sensed data from historical maps and high resolution photogrammetry was collected utilising a methodology similar to that of Warner and Steinmaus (2005) and García Torres et al. (2008). This facilitated the production of a traditional orchard habitat map (Natural England, 2012). Remotely sensing land-use characteristics is a technique becoming increasingly feasible and widely applicable due to its high accuracy and wide spatial footprint (Turner et al., 2003). However, errors are associated with this method (Nagendra et al., 2013); therefore ground-truthing of selected sites was also completed. During this truthing some original sites proved to be incorrectly identified; in addition sites which were also considered to be traditionally managed, but not identified by the survey were encompassed.
Data collection
Sampling Framework
Data collection occurred from late June to mid-July. This time period allowed for a sample encompassing the time of highest activity for G. nobilis adults (Whitehead, 2003). The time of sampling, however, is inconsequential for larval identification as frass produced by G. nobilis remains in situ for extended periods of time, allowing for the year round identification of G. nobilis populations (Hurt and Burrough, 2009). Although not directly measured, observations of adults in orchards aided the location of potential populations.
The number of trees sampled in each orchard was not uniform. The minimum number of trees suitable was derived from Burrough and Robertson (2008); providing a lower boundary of five. An initial habitat survey was completed for all orchards; within this, the basic orchard condition was assessed alongside a calculation of tree numbers. Due to the quantity of trees in some orchards a sub-sample was completed. In smaller orchards however all trees were assessed.
Sub-sampling consisted of a systematic spatial framework, which provides comprehensive and unbiased samples of red-listed populations (Hedgren and Weslien, 2008). The framework was derived from the quantity of trees within each site; produced from the initial habitat survey. This allowed for the calculation of a multiplicative factor which enabled a method to sample 40 trees. This schedule spatially segregated each orchard into quarters (e.g. Timmer et al., 1988), within which ten trees were assessed. Due to the high heterogeneity and variability of decay habitat (Pyle and Brown, 1999) a representative sample of microhabitats was also completed. The sample size was dependent on relative volume of decay habitat within each orchard. Therefore in well-developed orchards, with large volumes of microhabitats, sample sizes were larger. Overall, 20 orchards were sampled with a total of 478 trees and 122 microhabitats.
G. nobilis identification
Contemporarily, there is no completely unbiased sampling technique which accurately encapsulates distribution, abundance and richness of saproxylic communities (Ranius and Jansson, 2002). In order to accurately detail the population distribution of near-threatened and endangered species large sample sizes of more than 200 individuals (adult lifestages) are generally required (Martikainen and Kouki, 2002). Samples of this magnitude are potentially deleterious for often vulnerable G. nobilis populations. Furthermore, trapping methods do not always provide an accurate spatial sample of saproxylic populations (Ranius and Jansson, 2002; Alinvi et al., 2007; Jonsell and Weslien, 2003; Siitonen, 1994; Økland et al., 1996). Lastly, G. nobilis adults are notoriously elusive (PTES, 2008a). Therefore it was unfeasible to sample adult populations within this study.
Nonetheless, comprehensive methodological frameworks exist for sampling Coleopteran larvae. Larval instars of saproxylic species are obligate to decaying wood (Webb et al., 2008); providing a less dynamic and more reliable indicator of saproxylic beetle populations (Ranius and Nilsson, 1997). Furthermore, larvae sampling can too provide accurate samples of population structure and dynamics (Siitonen, 1994). Therefore within this dissertation the identification of larval populations took place. The dominant method of identifying G. nobilis larvae is microhabitat sampling. This method relies upon the identification of frass; a characteristic excrement deposited in the wake of feeding larvae (Hurt and Burrough, 2009). This method is based on the fact that G. nobilis is the only species to produce this distinctive frass within the traditional orchard habitat (Whitehead, 2003; Hopkins and Kirby, 2007; Macdonald et al., 2007). Beetle fragments were also used alongside frass as indicators of inhabitance (Ranius, 2000).
Multiscalar characteristics
Multiple techniques were used in order to collect characteristic data. The primary method of landscape analysis utilised a GIS. Remote sensing of landscape characteristics within 1km of sites was completed, with the manual digitisation providing the basis for land-use classification. Altitude and aspect were calculated for each site using a DEM derived from satellite altimetry data (Sharma and Panigrahy, 2007). The majority of qualitative data was compiled using likert scales with descriptive characteristics categorising variables.
The assessment of orchard characteristics took place predominantly through a semi-quantitative habitat evaluation (e.g. Tikkanen et al., 2007). Tree density was calculated using the orchard habitat perimeters provided by Natural England (2012) and/or GPS data collected during sampling.
Tree characteristics were composed of a series of quantitative variables. Recording the location of inhabited trees/orchards is a necessary procedure for G. nobilis surveying, allowing for representations of distribution within habitats (Dominy and Duncan, 2001). Tree species identification through phenotypic observation is a long and often difficult process (Wünsch and Hormaza, 2002); accordingly, sites with little information received taxonomic identification to genus. Inhabited trees were classed as those which provided accessible sample features, with the presence of G. nobilis indicators.
G. nobilis larvae reside deep within heartwood, therefore the direct sampling of microhabitat characteristics could be extremely damaging. Thus easy to measure, external features reflecting the conditions within microhabitats were utilised. Primary measurements comprised two major microclimatic variables identified by Bässler et al. (2010); temperature and humidity. External and internal paired readings were recorded; providing a comparison, and accounting for macro-climatic fluctuations. A further temporal study of temperature utilising a Tinytag™ monitor at a specific inhabited microhabitat within Tiddesley orchard. Qualitative measurements focused on the composition of wood mould, again utilising likert scales.
Results
Data collected from the field was first explored to allow for the avoidance of statistical errors related to the utilisation of incorrect statistical analyses and other common problems. A framework similar to that of Zuur et al. (2010) was used in order to assess data preceding statistical analysis. Normality and collinearity were of particular concern for parametric tests, in particular logistic regressions completed on each scale of habitat required independent variables. Correlation matrices were produced in each instance; highlighting interconnections between variables which may have been assumed as discrete. The removal of variables significantly related to one or more other variables excluded multicollinearity from the model whilst preserving an explanation of inhabitance.
Ten out of the twenty sampled sites displayed indicators of G. nobilis presence. Sites were significantly dispersed across the landscape, however, a number of inhabited and uninhabited sites are located within close proximity to one another.
Landscape characteristics
Ambiguity was found amongst some quantitative measures of landscape characteristics. Orchard aspect and altitude were not significantly different between inhabited and uninhabited sites (t=-1.389, df=9, p=0.198 and t=-0.183, df=9 p=0.859 respectively). Further to this, there was no significant correlation between said factors and the abundance of G. nobilis indicators within inhabited sites (rs=0.286, p=0.221 and rs=-0.14, p=0.954 respectively). Therefore it can be seen that topographical characteristics maintain little importance in G. nobilis distribution.
An analysis of land-use surrounding sites provided significant relationships with the presence of G. nobilis. The dominant factor was the volume of orchard habitat surrounding sites; occupied and unoccupied sites differed significantly (t=-2.985, df=9, p=0.15). Not only was the percentage of surrounding orchard land-use important for distribution of G. nobilis over sites, it also appeared to affect the abundance of inhabited trees, with a positive relationship, however this was discovered to be statistically insignificant (rs=4.16, p=0.068). Both urban and open ground volume displayed a negative (although insignificant) influences on abundance (rs=-0.340, p=0.198 and rs=-0.345, p = 0.137 respectively). Remotely sensed data although subjective, exhibited interesting relationships between landscape characteristics and G. nobilis presence, which supported statistical data.
The landscape surrounding a particularly suitable orchard (site 14) has high densities of both mixed woodland and orchard habitats (16.14 and 27.18 ha respectively), confirmed by a previous statistical correlation derived from these digitised land-use characteristics. Representations of openness allowed for a combined analysis with a finer scale characteristic, orchard size. With larger sites it appears there is a observable positive relationship with openness, however smaller sites appear to display a negative relationship with open ground.
The orchard sites sampled were generally fragmented and isolated; not linked with suitable habitat across the Worcestershire landscape. Although, some inhabited sites are well connected with orchard land-use and inhabited orchards, albeit over a relatively small scale. Distinct barriers inducing fragmentation were apparent across the landscape.
Conurbations were observed in close proximity to the majority negative sites. Historical data, helped to display the increasing urban sprawl derived from the increase in industry and agricultural within the Midlands (Wrigley, 1985); which appears to negatively impact the distribution of G. nobilis with sites located in close proximity generally remaining uninhabited, with a strong positive correlation between distance from urban areas and G. nobilis populations (rs=0.551, p=0.012). Orchard age was also inferred from historical maps. Only inferences can be made from such data, however, it appears that sites with historically persistence orchard land-use (e.g. where orchards have been present since the 1885) maintain contemporary populations of G. nobilis. Roads were too a dominant feature surrounding uninhabited sites. Three inhabited sites were discovered to the north of the A44, yet sites in close proximity, but to the south of the road were uninhabited.
Due to several significant relationships found between landscape characteristics and G. nobilis distribution a binary logistical regression was completed to assess which characteristics most likely predicted occupancy of sites. The regression displayed one significant predictor; the percentage of orchard land-use surrounding sites. This increased the predictive power of the null model (Nagelkerke r2=0.421); presenting a greater likelihood of colonisation with increasing volumes of orchard in the surrounding landscape.
Orchard characteristics
In general, orchard characteristics did not significantly influence G. nobilis distribution. The diversity of orchard tree species was not significantly different between inhabited or uninhabited sites (t=-0.889, df=9, p=0.397) and only a weak, insignificant correlation existed with the presence of G. nobilis (rs=-0.059, p=0.806). Tree density, quantity and habitat size displayed relatively weak and insignificant relationships with the presence of G. nobilis (rs=0.047, p=0.845 and rs=0.388, p=0.091, rs=0.373, p=0.105 respectively). Having completed Q-Q plots environmental variables, it was found that the quantity of trees was abnormally distributed and thus a logarithm was utilised to induce normality.
The form of management found within the orchard, though not displaying any statistical significance, appeared to play a vital role in extremes situations. Sites which were overgrown, with high abundances of species such as Ivies hedera were completely uncolonised by G. nobilis (Site 3 and 4), whereas sites with traditional management, either through periodic low intensity mowing or grazing appeared more suitable, with 60% of traditionally mown or grazed sites being occupied.
Tree characteristics
The characteristics found within tree habitat appear to play a more significant role in G. nobilis colonisation. Several relationships were found between tree characteristics and G. nobilis distribution. Prunus domestica facilitated the greatest G. nobilis populations (29.98% of records), relatively high compared to that of Prunus insititia (8.69%), Pyrus domestica (7.21%), and Malus domestica (1.51%). However a large proportion of inhabited data were collected from Site 11, composed of solely P. domestica. Therefore other factors influencing distribution may be obscured by this observation.
Both tree height and girth were found to be insignificantly different between inhabited and uninhabited sites (t=0.046, df=55, p=0.963; and t=0.776, df=55, p=0.447 respectively). No indicators of G. nobilis were discovered in tree girths less than 0.45m; with the mean tree girth per orchard showing that occupancy occurred in sites averaging between 0.5m and 1.5m. The majority of uninhabited records were provided by living trees early in their decomposition, whereas inhabited trees typically displayed heavily decaying boughs and trunks (75% of records), with 36.6% of moribund trees inhabited. Therefore there appears a greater likelihood of inhabitance in latter, mature stages of decomposition. Furthermore, orchards with greater densities of these late successional trees exhibited higher likelihood of colonisation (73.29% late successional trees in occupied sites). Not only was the stage of decay important so too was the quantity, with the density of decay microhabitats displaying a significant difference between inhabited and uninhabited trees (t=-13.490, df=81.944, p=<0.001).
A binary logistical regression was performed, utilising particularly significant habitat characteristics. The model showed that the density of microhabitats and tree species were significant factors influencing colonisation of tree habitats. The density of microhabitat increased the probability of G. nobilis presence by over three times in trees with fine substrate (exp b= 3.707). Within the category of tree genera Malus displayed the greatest significance, yet did not drastically alter the probability of presence. The model provided a reasonable fit overall, and allows for adequate prediction of inhabitance (Nagelkerke r2=0.417).
Microhabitat characteristics
Monitoring temperature provided an insight into the microclimatic stability produced within microhabitats and orchards to a greater extent. The range of measurements found in internal temperatures was significantly less than that of external temperature (Internal=14.80, External=15.70). This is supported further by measurements recorded from an individual tree feature within Site 11. However, a distinct diurnal relationship between internal and external temperatures persists. Within the morning hours (6:00-12:00) internal temperature falls below that of external temperature.
The range of humidity recorded was far more variable between individual features (Internal=47.80 and External=32.30). Nevertheless, higher mean moisture levels were recorded internally (mean=61.06%) compared to the externally (mean=55.48%). Therefore it can be seen that distinct microclimates are created internally within trees. An independent Mann-Whitney U Test was completed for both temperature and humidity within inhabited and uninhabited features. This showed significant difference between the two data sets, thus rejecting the null hypothesis (Temperature: p=<0.001; Humidity: p=0.044). This highlights the difference between microhabitats found within inhabited and uninhabited orchards.
Feature orientation and depth of rot helped to display potential microclimatic conditions. However, the orientation of microhabitat entrances was not significantly different between inhabited and uninhabited sites (t=-1.458, df=120, p=0.147). Therefore the orientation of features appears insignificant for G. nobilis distribution. A subjective analysis does appear to show that easterly facing features are less well inhabited; nevertheless the variability of results limits the significance of such interpretations. On the other hand the depth of the feature does appear to play an important role in determining distribution. Inhabited trees has significantly deeper features than those which were uninhabited (t=-8.728, df=120, p=<0.001).
Internal temperature and depth of rot were collinear with several other variables and were thus removed from the logistic regression model. Wood mould density was the dominant factor influencing the occurrence of G. nobilis. However, temperature also appears to influence distribution, with higher temperatures providing a greater likelihood of G. nobilis presence. The Nagelkerke r2 value indicates that the model accurately predicts 70.7% of variation, thus strongly predicting presence. Humidity was also implicated within the model, however, results were unaccountably variable throughout the sampling period, and thus were discounted as a significant variable influencing G. nobilis presence.
Discussion
The principal objective of this dissertation was to elucidate differences in multi-scalar habitat characteristics between orchards both inhabited and uninhabited by G. nobilis in order to provide evidence concerning variables determining presence. These variables are essentially located at either landscape or habitat scales (Mazerolle and Villard, 1999; Stoner and Joern, 2004; Tikkanen et al., 2006). Within this study three separate spatial scales were classified within habitat: 1) microhabitat, 2) tree, 3) orchard; with a single scale representing the landscape (01).
Several significant characteristics were displayed at both habitat and landscape scales (Figure 5.1). The fact that specific characteristics are found within inhabited sites suggests that there is distinct difference between the characteristics of orchards exhibiting G. nobilis presence and absence. When compared, habitat characteristics explained a greater amount of probability associated with the presence of G. nobilis this helps to answer the second research question proposed within this study. Relationships at habitat level were strongest at microhabitat scale, shown by the high prediction success of the microhabitat characteristics (70.7%). Nevertheless landscape influences were observed influencing the presence of G. nobilis; with the volume of orchard habitat in the landscape provided relatively accurate predictions of presence. The results displayed within this study highlight several key characteristics located at both scales suggesting a balance between these scales determines the presence of G. nobilis. Each scale is discussed in turn, proceeding with the most influential.
Habitat / Site scale
Microhabitat characteristics
Binary logistic regression displayed a particularly accurate model for the prediction of G. nobilis presence, derived predominantly from microhabitat characteristics. Within this model the density of the wood mould was identified as being a strong predictor. Output showed that G. nobilis was present in trees which displayed lower densities of wood mould. A reduction in substrate size and density over time indicates the presence of significant processing and decomposition within habitats (Mattson et al., 1987); thereby inferring that a prerequisite of G. nobilis presence is well processed substrate within microhabitats. As of yet little work has been completed for G. nobilis at a microhabitat scale, yet similar relationships with fine substrate have been detailed by several studies of saproxylic species (Nilsson and Baranowski, 1997; Abrahamsson and Lindbladh, 2006). From such studies it is apparent that certain species, and potentially G. nobilis, require pre-processing in order for assimilation of nutrients (Kaila et al., 1994; Butler et al., 2002; Jansson et al., 2009).
Wood mould density and processing is influenced by several factors. The wood mould density within this study was significantly correlated to the successional stage of the tree habitat (r²=0.539, p=<0.001). This relationship corroborates findings from Nilsson et al. (2002) and Ranius (2003), who stated that dead wood is a product of stand age, low management and low disturbance. Colonisation of fungi was not analysed within this study, yet it may be important, with observations suggesting microorganisms assist processing of decomposing wood material, thereby reducing wood mould density (Jonsell et al., 2005; Djupström et al., 2010). In some cases fungal processing is so significant co-variation has been suggested (Similä et al., 2006). Therefore further analysis of fungal composition may provide relationships with G. nobilis.
Enhanced fining of decomposition was also related to rot depth within the orchard microhabitat (rs=0.42, p=<0.001) and although not included as a variable within logistic regression, rot depth displayed a strong positive relationship with G. nobilis presence. G. nobilis larvae specifically are observed habituating rot deep within the heartwood of the tree (Alexander, 2008b). Substrate characteristics have yet been quantified for G. nobilis, however, they are important for several saproxylic species (Jacobs et al., 2007). The combinations of density and depth observed within this study have been identified previously for similar species (Nilsson and Baranowski, 1999). It is therefore likely that multiple associated substrate characteristics influence the presence of G. nobilis.
Microclimatic characteristics established within microhabitats also influenced presence of G. nobilis within this study. Likelihood of presence coincidentally increased with temperature; furthermore, there was significant difference between temperatures in inhabited and uninhabited microhabitats. Research completed by Renault et al. (2005) indicates that G. nobilis exhibits a low cold tolerance, therefore supporting this finding. Moreover, temperature regulation is important within this study; indicating the low variability of microhabitat temperatures compared to external, macroscale fluctuations. The regulation of temperatures at this scale is seen to increase species abundance in saproxylic assemblages (Wright, 1983; Lindo et al., 2008; Lachat et al., 2012) as well as influencing the presence of G. nobilis.
The regulation of moisture is also essential for some species (Jonsell et al., 2001; Chiari et al., 2012). However, a complicated relationship between microhabitat moisture and G. nobilis was present. Repeat measurements display greater internal variation compared to external conditions; although data from an extended period displayed a high mean with relatively low deviation (68.22 ± 13.43%). Furthermore, moisture was utilised in a model predicting G. nobilis presence. As shown by Hjätlén et al. (2007) moisture content of wood significantly affects the rate of decomposition as well as the composition of substrate; both of which have been previously shown to influence G. nobilis presence within this study. The regulation provided by microhabitats may have indirectly affected G. nobilis facilitating optimum substrate conditions, whilst reducing mortality from extreme climatic variation.
Orientation of microhabitat features often influences microclimate, and therefore has been observed displaying a relationship with the presence of saproxylic species (Ranius and Nilsson, 1997; Ranius and Wilander, 2000; Ranius, 2002a). Nevertheless there was no association displayed between G. nobilis and the orientation of features in this study. This may represent the depth at which G. nobilis larvae are found within heartwood decay (Whitehead, 2003), allowing for reduced external influences. This is supported by the absence of relationships between G. nobilis and other external influences such as macroscale temperature and humidity. A further possible explanation lies at a broader spatial scale; the tree density of traditional orchard cultivation. Studies completed previously analyse the presence of saproxylic species in closed canopy, dense woodland, which generally exhibited low light penetration; creating significant implications for orientation on radiative heating. The setting of this study allows for greater light penetration due to regulated planting densities (Alexander, 2008b), therefore potentially reducing the influence of orientation.
Previous studies have generally neglected characteristics at microhabitat scales. However within this study microhabitat characteristics significantly influenced G. nobilis presence. Therefore, further study of is essential, with strong preliminary evidence suggesting relationships at this scale.
Tree characteristics
A key result of this study was the absence of significant relationships between tree height, girth and G. nobilis presence. The same result was displayed by Alexander and Bower (2011, Table 1) with a similar range of girths inhabited. Nevertheless, due to previously inconsistent findings and the significant obligation to specific tree girths displayed by many European saproxylic species (Økland et al., 1996; Siitonen, 2001; Lindhe et al., 2005; Gibb et al., 2006; McGeoch et al., 2007; Buse et al., 2008; Müller and Bütler, 2010; Horák et al., 2010), it was expected that a relationship might exist. A range of potential relationships exist from studies of similar species (Martikainen et al., 2000; Ranius and Jansson, 2000; Schiegg, 2001; Ranius, 2002a; Hammond et al., 2004; Penttilä et al., 2004; Yee, 2005; Buse et al., 2007; Oleska et al., 2007; Wu et al., 2008). However, no clear relationship with girth was identified for G. nobilis (cf. Alexander and Bower, 2011). Conversely, no distinct lower boundary was observed as in their study, yet they did recognise complications preventing the identification of consistent relationships, e.g. the influence of tree species (Weedon et al., 2009).
The density of microhabitat and thus availability of decay substrate significantly affected the presence of G. nobilis. The significance of this relationship has been previously highlighted (Whitehead, 2003; Alexander. 2008b; Lush et al., 2009). Grove (2002b) in particular displays a strong relationship between veteran features and saproxylic presence, further suggesting the use veteran features as signposts for saproxylic species. Veteran features are crucial within this study as they are inherently linked to the age and condition of trees (Hopkins et al., 2005; Fay, 2002; Alexander, 2008b) both of which have been identified important for G. nobilis (Whitehead, 2003; Alexander, 2008b). The observed effectiveness of veteran features as indicators of presence surrounds the associated creation of suitable habitat for saproxylic species.
As previously described the availability of substrate was an essential feature for occupied habitat in south Worcestershire. However, quality, composition and form also appear influential within this study. This is similar to influences on the presence of other saproxylic species (Jacobs et al., 2007). The composition of decay within this study was measured through an assessment of tree successional stage. During the sequence of tree development, heartwood dies and woody material decays (Boddy and Rayner, 1983). The quality and composition of wood material, as perceived by G. nobilis will vary alongside development and decay (Alexander, 2001). Beetles are particularly sensitive to these changes (Araya, 1993; Jukes et al., 2002; Sverdrup-Thygeson, 2001) and within this study there was an increased presence associated with rot found in mid-to-late successional trees; with only completely living trees displaying absence of G. nobilis. Similar studies assessing this relationship have provided comparable results (Rukke, 2000; Hammond et al., 2001; Yee et al., 2006; Ranius et al., 2009b; Brunet and Isacsson, 2009a; Lassauce et al., 2011; Russo et al., 2011).
The influence of tree species within this study was complex and contradictory. Tree species was included in the logistic regression as a factor influencing G. nobilis presence; with Prunus providing particularly significant influence. Previous research also provides contradictions with regards to the presence or absence of tree species associations. Whitehead (2003) alongside Evans and Whitehead (2005) observed obligations to the genus Prunus, with specific species being particularly favourable (e.g. Purple Pershore). This is reinforced by relationships found in other studies with similar saproxylic species (e.g. Lachat et al., 2006; Oleska et al., 2007 Davies et al., 2008; Dubois et al., 2009; Müller and Goßner, 2010). Yet other research regarding G. nobilis supports the latter conclusion with presence observed in the majority of common orchard cultivars (Hurt and Burrough, 2009; Alexander, 2008b; Lush et al., 2009). Several other studies regarding saproxylic species also complement this conclusion suggesting that it is the individual tree characteristics rather than tree species that are dominant in influencing the presence of saproxylic species (Warren and Key, 1991; Ulyshen and Hanula, 2009). As inferred by Alexander and Bower (2011), the production of wood and decay material is affected by the tree species. Therefore the perceived influence of tree species may be as a consequence of indirect influences on substrate characteristics.
At the tree habitat scale many associations found appear to translate to microhabitat characteristics; influencing characteristics at a finer scale. The majority of results display a distinct link to the volume or composition of wood decay within tree habitats. Quantification of relationships has provided novel insights, suggesting that the substrate requirements at habitat scales are of primary importance.
Orchard characteristics
Results from this study indicated no significant difference between inhabited or uninhabited orchards with regards to several characteristics often assumed to influence the presence of saproxylic species. Both size and quantity of trees displayed directional yet, statistically insignificant relationships. Small sites throughout the landscape remained inhabited, thereby accounting for the absence of relationships. Habitat size does not significantly influence the presence of a number of saproxylic species (Irmler et al., 2010); however these species are observed displaying strong dispersal abilities. Other less mobile, saproxylic species within patch-matrix habitats, were generally more often present in larger habitats (Murcia, 1995; Bender et al., 1998; Sadler et al., 2006; Similä et al., 2006; Sahlin and Schroeder, 2010). Results provided in this study therefore contradict contemporary research which suggests somewhat limited dispersal (Whitehead, 2003). Inferring high dispersal ability allows relationships to be effectively explained, with G. nobilis utilising other proximal habitat with the landscape. Such patch and matrix utilisation has facilitated weak relationships between presence and habitat size in other studies of saproxylic species (Debinsky and Holt, 2000). This opposes the common assumption that the species is restricted to traditional orchard (Alexander, 2008b), potentially frequenting external habitats during active periods.
The density of trees is loosely regulated through the utilisation of traditional orchard management (Burrough and Robertson, 2008); nevertheless significant variation was still present across sampled habitats. Contrary to contemporary saproxylic research (e.g. (Hindmarch and Reid, 2001; Lindhe et al., 2005; Johansson et al., 2007; Horák et al. 2012) densely cultivated sites within this study supported populations. Not only does this contradict said research, it also conflicts previous studies, which suggest that dense cultivation prevents G. nobilis colonisation (Burrough and Robertson, 2008). Relationships are explained by generally high incidences of snags and dead limbs within habitats. These successional features exhibit low canopy cover, and thus minimally perturb sunlight penetration and larvae incubation (Vernon and Vannier, 2001; Müller and Brandl, 2009), factors previously associated with density-absence relationships. This indicates a reduced influence of density due cultivated traditional orchards.
The age of orchards was inferred from historical records of orchard land-use; therefore direct quantitative relationships were not present. All sites supporting contemporary G. nobilis populations were located within areas of continuous, historical orchard land-use. Alexander (2008b) too highlighted the importance of temporal persistence in land-use for G. nobilis. Such persistence appears to relate to decay suitability through increasing microhabitat density and diversity with is concomitant with age (Warren and Key, 1991; Regnery et al., 2013) whilst also facilitated in part by traditional management. Larger scale landscape persistence may also influence presence (Hultberg et al., 2010), yet this was not quantified. This may explain the loss of G. nobilis from many regions due to the poor response to rapid habitat modification (Evans and Whitehead, 2005). This may also explain a number of previous relationships, with persistence as well as presence of a habitat characteristics thereby appearing crucial.
Despite the perceived importance of specific traditional management techniques (Alexander, 2008b, PTES 2008b; Lush et al., 2011) there was no relationship between presence and methods of traditional management. Although, sites which were unsuitably managed (both intensive and derelict) did not support G. nobilis populations. Dominance of floral competition (Jansson, 2009) as a result of insufficient canopy management (Horák and Rébl, 2013) may directly slow the growth of trees (Merwin and Stiles, 1994), and thus hinder transition through the successional sequence. This dense orchard flora also negatively impacted beetles through the perturbation of flight and dispersal (Dubois and Vignon, 2008) in turn reducing resource utilisation. The balance of cultivation and low intensity management therefore regulates conditions for G. nobilis.
Landscape scale
Although significant associations have been found with habitat characteristics at the previous scales, the landscape may still provide a broad influence on the distribution and presence at subordinate scales (e.g. Tscharntke et al., 2005). Landscape characteristics may ultimately influence the availability of local habitat (Dauber et al., 2005) and therefore require consideration.
Landscape characteristics
A single significant statistical association was derived from landscape characteristic analysis: Increases in the percentage of orchard land-use within 1km of inhabited sites increased the probability of G. nobilis presence. Other statistical associations provided by landscape variables were insignificant and weakly related. In general, if organisms are not influenced by landscape characteristics then they may exhibit strong dispersal (Kouki et al., 2012; Lizeé et al., 2012). Therefore the absence of multiple significant landscape associations with G. nobilis indicates the potential for dispersal across the landscape. This further supports evidence within this study contradicting the previous dispersal assumptions provided by Whitehead (2003). This observation of greater dispersal for G. nobilis is also supported by Szacki (1999), who showed that the dispersal of small organisms is often underestimated.
The relationship between presence and volume of proximal orchard habitat has a number of implications for G. nobilis. Results infer that connectivity between orchard habitats must be maintained in order for G. nobilis populations to persist. Habitat connectivity is especially important in these heterogeneous landscape matrices due to the scarcity of suitable habitat, with high connectivity affording diversity and abundance (Östman et al., 2001; Chisholm et al., 2011). Several factors are associated with maintaining connectivity within south Worcestershire. Habitat corridors are one such factor. These linear features connect sites across south Worcestershire. In this instance, corridors allowed for the connection of several inhabited orchards, potentially facilitating dispersal of G. nobilis across the landscape and providing the inhabited clusters identified. Further analysis also showed that sites without indicators of G. nobilis were generally isolated from other suitable habitat, and not connected by corridors of suitable habitat; inferring impacts of habitat fragmentation (Tscharntke et al., 2002; Fahrig, 2003).
In many cases it appeared that isolation was a result of low matrix permeability. Landscape permeability (e.g. Kindlmann et al., 2005; Dubois and Vignon, 2008) is a function of the surrounding habitat matrix (Wagner and Fortin, 2005) and is therefore a product of connectivity and fragmentation. Within south Worcestershire sites surrounded by large areas of arable fields appeared to provide a suitable matrix for dispersal and subsequent habitat colonisation. This lead to the clustering of inhabited sites around regions with generally low vegetation height in matrices and suitable orchard habitat. Nevertheless, small sites situated in such ‘open’ landscapes did not support G. nobilis populations. In these instances it appears that increased exposure derived from open landscape matrices facilitates increased negative, climatic influence on orchards (Chen et al., 1995).
An attempt at estimating exposure was completed through analysis of topography and aspect of the surrounding landscape. However, both features were insignificantly different between inhabited and uninhabited sites, with low topographical variation across the entire sample region (±12.21m). Previous research on saproxylic assemblages displayed significant associations with landscape topography (Franc et al., 2007), however topographic variation was far greater; potentially providing observed impacts. Furthermore, the impact of these factors is determined by climatic conditions, with water availability determining their relative influence (Gallardo-Cruz et al., 2009).
Lastly, isolation and loss of connectivity was also induced by the process of urbanisation across the landscape; with urban land-use introducing further opposition to dispersal. Urban areas severely perturb movement of organisms across the landscape (Gustafson and Gardner, 1996; Kindlmann et al., 2005; Ódor et al., 2006; Hedin et al., 2008). Contemporary studies relate the absence of saproxylic species from habitat to urban landscape barriers inhibiting landscape permeability (Bolger et al., 2000; Gibb and Hochuli, 2002; Brunet and Isacsson, 2009b; Bailey et al., 2010). The most significant landscape barrier influencing presence appears to be that of roads. As detailed in Bhattacharya et al. (2003) roads disrupt habitat and prevent the movement of organisms across the landscape. Inhabited and uninhabited sites are proximal in Worcestershire, this juxtaposition appears to be a result of road infrastructure preventing the dispersal and establishment within certain sites.
The surrounding landscape provided a complex of interactions, with several concepts associated with metapopulation dynamics (Levins, 1969) and patch-matrix landscapes appearing dominant. However, firm conclusions surrounding the impact of the landscape are hindered by a lack of ecological and functional knowledge with regards to G. nobilis. Increases in ecological knowledge will facilitate a better understanding of the controls of habitat characteristics across all scales.
Conclusions
This exploratory research has highlighted several important relationships between G. nobilis and habitat characteristics spanning multiple spatial scales. Many of these relationships encompass characteristics previously unconsidered for G. nobilis. Two spatial scales dominated the presence of G. nobilis within south Worcestershire. Whilst uninhabited sites displayed few of the common features found in inhabited orchard habitats, implying that intra-habitat characteristics ultimately determine G. nobilis distribution.
Habitat scale characteristics were particularly influential. Within the microhabitat scale, temperature and humidity were defining factors, providing both direct and indirect influences on G. nobilis. The nature of the decay substrate and microclimates found at this scale appear particularly important, yet these characteristics are particularly hard to replicate through conservation. However, the multi-scalar nature of habitat characteristics allows for the production of desired fine scale characteristics through alterations in broader scales. Tree scale associations were dominated by several characteristics which inferred the quality and quantity of decay habitat within sites. Across the orchard scale no significant relationships were observed. Yet, the intermediate levels of management generally found within inhabited orchards provided suitable habitat for G. nobilis.
At the landscape scale associations exhibited were generally weak. The amount of orchard landscape proximate to sites was influential. However, the nature of their influence is dependent on the dispersal ability of G. nobilis. Information regarding this ability remains vague, therefore the importance of landscape characteristics for G. nobilis could be obscured due to this deficit of knowledge.
This multi-scalar, interrelated influence of habitat characteristics contrasts previous research observing influences derived from relatively discrete factors. However, the relative influence of each scale was significantly different. It therefore is apparent that G. nobilis presence is affected to a greater extent by habitat conditions, as opposed to the landscape composition proximal to sites.
References
Abrahamsson M. and Lindbladh M. (2006) A comparison of saproxylic beetle occurrence between man-made high- and low-stumps of spruce (Picea abies), Forest Ecology and Management, Vol. 226(1), 230-237.
Alder D. and Synott T J. (1992) Permanent sample plot techniques for mixed tropical forest, Tropical Forestry Papers, Vol. 25, 77-80.
Alexander K N A. (1998) The links between forest history and biodiversity: the invertebrate fauna of the ancient pasturewoodlands in Britain and its conservation, in Kirby K J W (ed), The ecological history of European history, Cab international, New York, 73-80.
Alexander K N A. (1999) The invertebrates of Britain’s wood pastures, British Wildlife, Vol. 11(2), 108-117.
Alexander K N A. (2001) What are veteran trees? Where are they found? Why are they important?, in Read H., Forfang A. S., Marciau R., Paltto H., Andersson L. and Tardy B. (eds), Tools for preserving woodland biodiversity, Textbook 2, NACONEX, Sweden, 28-31.
Alexander K N A. (2002) The Noble Chafer Gnorimus nobilis in Worcestershire – a report on the 2002 survey, Unpublished report for The People’s Trust for Endangered Species.
Alexander K N A. (2003) The British saproxylic invertebrate fauna, Proceedings of the second pan – European conference on saproxylic beetles, PTES, London.
Alexander K N A. (2008a) Tree biology and saproxylic coleoptera: issues of definitions and conservation language, Revue d’Ecologie (la Terre et la Vie), Vol. 63, 1-5.
Alexander K N A. (2008b) The Special Importance of Traditional Orchards for Invertebrate conservation, with a Case Study of the BAP Priority Species the Noble Chafer Gnorimus nobilis, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 12-18.
Alexander K N A. (2010) Saproxylic beetles, in Newton A C. (ed.) Biodiversity in the New Forest, Pisces Publications, Newbury, 46-53.
Alexander K N A. and Bower L. (2011) The Noble Chafer and traditional orchards – an old growth species in the English cultural landscape, British Wildlife, Vol. 23(1), 17-22.
Alinvi O., Ball J P., Danell., Hjältén J. and Pettersson R B. (2007) Sampling saproxylic beetle assemblages in dead wood logs: comparing window and eclector traps to traditional bark sieving and refinement, Journal of Insect Conservation, Vol. 11, 99-112.
Araya K. (1993) Relationship between the decay types of dead wood and occurrence of Lucanid beetles, Applied Entomology and Zoology, Vol. 28(1), 27-33.
Atkinson G. and Winnall R A. (2008) Rejuvenating Traditional Orchards, How Multidisciplinary Landscape Partnership Schemes Can Serve as a Vehicle for Restoration – Wyre Forest, West Midlands, United Kingdom, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 19-22.
Bailey D., Schmidt-Entling M H., Eberhart P., Herrmann J D., Hofer G., Kormann U. and Herzog F. (2010) Effects of habitat amount and isolation on biodiversity in fragmented traditional orchards, Journal of Applied Ecology, Vol. 47(5), 1003-1013.
Barker S., Burrough A., Cordrey L., Merry K. and Wedge C. (2011) Conserving the wildlife of traditional orchards, British Wildlife, Vol. 23(1), 8-16.
Bässler C., Müller J., Dziock F. and Brandl R. (2010) Effects of resource availability and climate on the diversity of wood-decaying fungi, Journal of Ecology, Vol. 98, 822-832.
Ben-David M., Blundell G M., Kern J W., Maier J A K., Brown E D. and Jewett S C. (2005) Communication in river otters: Creation of variable resource sheds for terrestrial communities, Ecology, Vol. 86(5), 1331-1345.
Bender D J., Contreras T A. and Fahrig L. (1998) Habitat loss and population decline: a meta-analysis of the patch size effect, Ecology, Vol. 79(2), 517-533.
Benton T G., Bryant D M., Cole L. and Crick H Q P. (2002) Linking agricultural practice to insect and bird populations: a historical study over three decades, Journal of Applied Ecology, Vol. 39(4), 673-687.
Berg Å., Ehnström B., Gustavsson L., Hallingbäck T., Jonsell M. and Weslien J. (1994) Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations, Conservation Biology, Vol. 8, 718-731.
Bergman K-O., Jansson N., Claesson K., Palmer M W. and Milberg P. (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles, Forest Ecology and Management, Vol. 265, 133-141.
Bhattacharya M., Primack R B. and Gerwein J. (2003) Are roads and railroads barriers to bumblebee movement in a temperate urban conservation area, Biological Conservation, Vol. 109, 37-45.
Bignal E M. and McCracken D I. (1996) Low-intensity farming systems in the conservation of the countryside, Journal of Applied Ecology, Vol. 33(3), 413-424.
Billeter R., Liira J., Bailey D., Bugter R., Arens P., Augenstein I., Aviron S., Baudry J., Bukacek R., Burel F., Cerny M., De Blust G., De Cock R., Diekötter T., Dietz H., Dirksen J., Dormann C., Durka W., Frenzel M., Hamersky R., Hendrickx F., Herzog F., Klotz S., Koolstra B., Lausch A., Le Couer D., Maelfait J P., Opdam P., Roubalova M., Schermann A., Schermann N., Schmidt T., Schweiger O., Smulders M J M., Speelsmans M., Simova P., Verboom J., Van Wingerden W K R E., Zobel M. and Edwards P J. (2008) Indicators for biodiversity in agricultural landscapes: a pan-European study, Journal of Applied Ecology, Vol. 45(1), 141-150.
Boddy L. and Rayner A D M. (1983) Origins of decay in living deciduous trees: the role of moisture content and a re-appraisal of the expanded concept of tree decay, New Phytologist, Vol. 94, 623-641.
Bolger D T., Suarez A V., Crookis K R., Morrison S A. and Case T J. (2000) Arthropods in urban habitat fragments in southern California: area, age and edge effects, Ecological Applications, Vol. 10(4), 1230-1248.
Bouchard P., Grebennikov V V., Smith A B T. and Douglas H. (2009) Biodiversity of Coleoptera, in Foottit R G. and Adler P H. (eds.) Insect Biodiversity: Science and Society, Wiley-Blackwell, Oxford, 265-301.
Boyatzis R E. (1998) Transforming Qualitative Information: Thematic Analysis and Code Development, SAGE publications, London.
Brown M W. and Schmitt J J. (2001) Seasonal and diurnal dynamics of beneficial insect populations in apple orchards under different management intensity, Environmental Entomology, Vol. 30(2), 415-424.
Brunet J. and Isacsson G. (2009a) Influence of snag characteristics on saproxylic beetle assemblages in a south Swedish beech forest, Journal of Insect Conservation, Vol. 13, 515-528.
Brunet J. and Isacsson G. (2009b) Restoration of beech forest for saproxylic beetles – effects of habitat fragmentation and substrate density on species diversity and distribution, Biodiversity and Conservation, Vol. 18(9), 2387-2404.
Burrough A. and Robertson H. (2008) Traditional Orchard Survey – Mapping England’s Traditional Orchards, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 26-31.
Buse J., Schröder B. and Assmann T. (2007) Modelling habitat and spatial distribution of an endangered longhorn beetle – A case study for saproxylic insect conservation, Biological Conservation, Vol. 137(3), 372-381.
Buse J., Ranius T. and Assmann T. (2008) An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer, Conservation Biology, Vol. 22(2), 329-337.
Butler J., Alexander K. and Green T. (2002) Decaying Wood: An Overview of Its Status and Ecology in the United Kingdom and Continental Europe, USDA Forest Service General Technical Report PSW-GTR-181, 11-19.
Cardoso P., Aranda S C., Lobo J M., Dinis F., Gaspar C. and Borges P A V. (2009) A spatial scale assessment of habitat effects on arthropod communities of an oceanic island, Acta Oecologica, Vol. 35(5), 590-597.
Chase J M. (2007) Drought mediates the importance of stochastic community assembly, Proceedings of the National Academy of Sciences of the USA, Vol. 104(44), 17430-17434.
Chen J., Franklin J F. and Spies T A. (1995) Growing-season microlcimate gradients from clearcut edges into old-growth Douglas-fir forests, Ecological Applications, Vol. 5(1), 74-86.
Chesmore E D. (2001) Application of time domain signal coding and artificial neural networks to passive acoustical identification of animals, Applied Acoustics, Vol. 62(12), 1359-1374.
Chesmore E. D. and Ohya E. (2004) Automated identification of field-recorded songs of four British grasshoppers using bioacoustic signal recognition, Bulletin of Entomological Research, Vol. 94, 319–330.
Chiari S., Lorenzo M., Audisio P. and Ranius T. (2012) Habitat of an Endangered Saproxylic Beetle, Osmoderma eremita, in Mediterranean Woodlands, Ecoscience, Vol. 19(4), 299-307.
Chisholm C., Lindo Z. and Gonzalez A. (2011) Metacommunity diversity depends on connectivity and patch arrangement in heterogeneous habitat networks, Ecography, Vol. 34(3), 415-424.
Cloke P. and Jones O. (2001) Dwelling, place, and landscape: an orchard in Somerset, Environment and Planning A, Vol. 33, 649-666.
Collinge S K. (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns, Ecology, Vol. 81, 2211–2226.
Cooter J., Dibb J R. and Walsh D B. (1991) A Coleopterists Handbook, Third edition, The Amateur Entomological Society, Feltham.
Cordrey L., Bullock D J., Barker S., Bouch D. and Groves C. (2008) Orchards in The National Trust: an Overview of their History, Economics, Wildlife and People, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 42-50.
Dauber J., Purtauf T., Allspach A., Frisch J., Voigtländer K. and Wolters V. (2005) Local vs. Landscape controls on diversity: a test using surface-dwelling soil macroinvertebrates of differing mobility, Global Ecology and Biogeography, Vol. 14, 213-221.
Davies Z G., Tyler C., Stewart G B. and Pullin A S. (2008) Are current management recommendations for saproxylic invertebrates effective? A systematic review, Biodiversity Conservation, Vol. 17, 209-234.
Debinsky D M. and Holt R D. (2000) A survey and overview of habitat fragmentation experiments, Conservation Biology, Vol. 14, 342–355.
Djupström L B., Perhans K., Weslien J., Schroeder L M., Gustafsson L. and Wikberg S. (2010) Co-variation of lichens, bryophytes, saproxylic beetles and dead wood in Swedish boreal forests, Systematics and Biodiversity, Vol. 8(2), 247-256.
Dominy N J. and Duncan B. (2001) GPS and GIS methods in and African Rain Forest: Applications to tropical ecology and conservation, Conservation Ecology, Vol. 5(2), Article 6.
Duarte F., Jones N. and Fleskens L. (2008) Traditional olive orchards on sloping land: Sustainability or abandonment?, Journal of Environmental Management, Vol. 89(2), 86-98.
Dubois G F. and Vignon V. (2008) First results of radio tracking of Osmoderma eremita (Coleoptera: Cetoniidae) in French chestnut orchards, Revue d’Ecologie, Terre et Vie, Vol. 10, 131-138.
Dubois G F., Vignon V., Delettre Y R., Rantier., Vernon P. and Burel F. (2009) Factors affecting the occurrence of the endangered saproxylic beetle Osmoderma eremita (Scopoli, 1763) (Coleoptera: Certoniidae) in an agricultural landscape, Landscape and Urban Planning, Vol. 91, 152-159.
Elith J. and Leathwick J R. (2009) Species Distribution Models: ecological explanation and prediction across space and time, The Annual Review of Ecology, Evolution and Systematics, Vol. 40, 677-697.
Epstein D L., Zack R S., Brunner J F., Gut L. and Brown J J. (2001) Ground Beetle Activity in Apple Orchards under reduced pesticide management regimes, Biological Control, Vol.21(2), 97-104.
Evans H C. and Whitehead P F. (2005) Entomogenous fungi of arboreal Coleoptera from Worcestershire, England, including the new species Harposporium bredonense. Mycological Progress, Vol. 4(2), 91-99.
Ewers R M., Thorpe S. and Didham R K. (2007) Synergistic interactions between edge and area effects in a heavily fragmented landscape, Ecology, Vol. 88(1), 96-106.
Fahrig L. (2003) Effects of habitat fragmentation on Biodiversity, Annual Review of Ecology, Evolution, and Systematics, Vol. 34, 487-515.
Farr I. and Chesmore D. (2007) Automated bioacoustic detection and identification of wood-boring insects for quarantine screening and insect ecology, Proceedings of the 4th International Conference on Bioacoustics, Vol. 29(3), 201–208.
Fay N. (2002) Environmental arboriculture, tree ecology and veteran tree management, Arboricultural Journal, Vol. 26(3), 213-238.
Fetridge E D., Ascher J S., Langellotto G A. (2008) The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea), Annals of the Entomological Society of America, Vol. 101(6), 1067-1077.
Fierke M K., Kinney D L., Salisbury V B., Crook D J. and Stephen F M. (2005) Development and comparison of intensive and extensive sampling methods and preliminary within-tree population estimates of Red Oak Borer (Coleoptera: Cerambycidae) in the Ozark Mountains of Arkansas, Environmental Entomology, Vol. 34(1), 184-192.
Franc N., Götmark F., Økland B., Nordén B. and Paltto H. (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest, Biological Conservation, Vol. 135, 86-98.
Frank D., Finckh M. and Wirth C. (2009) Impacts of land use on habitat functions of old-growth forests and their biodiversity, in Wirth C., Gleixner G. and Heimann M. (eds.) Old Growth Forests: Function, Fate and Value, Ecological Studies, Vol. 207, Springer, Berlin.
Franklin J F., Spies T A., Van Pelt R., Carey A B., Thornburgh D A., Berg D R., Lindenmayer D B., Harmon M E., Leeton W S., Shaw D C., Bible K. and Chen J. (2002) Disturbance and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir as an example, Forest Ecology and Management, Vol. 155(1), 399-423.
Gallardo-Cruz J A., Pérez-García E A. and Meave J A. (2009) β-diversity and vegetation structure as influenced by slope aspect and altitude in a seasonally dry tropical landscape, Landscape Ecology, Vol. 24(4), 473-482.
García Torres L., Peña-Barragán J M., López-Granados., Jurado-Expósito M. and Fernández-Escobar R. (2008) Automatic assessment of agro-environmental indicators from remotely sensed images of tree orchards and its evaluation using olive plantations, Computers and Electronics in Agriculture, Vol. 61(2), 179-191.
Gibb H. and Hochuli D F. (2002) Habitat fragmentation in an urban environment: large and small fragments support different arthropod assemblages, Biological Conservation, Vol. 106(1), 91-100.
Gibb H., Pettersson R B., Hjältén J., Ball J P., Johansson T., Atlegrim O. and Danell K. (2006) Conservation-orientated forestry and early successional saproxylic beetles: Responses of functional groups to manipulated dead wood substrates, Biological Conservation, Vol. 129(4), 437-450.
Groombridge B. (1992) Global biodiversity: status of the earth’s living resources, Chapman and Hall, London.
Grove S J. (2002a) Saproxylic insect ecology and the sustainable management of forests, Annual Review of Ecology and Systematics, Vol. 33, 1-23.
Grove S J. (2002b) Tree basal are and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics, Ecological Indicators, Vol. 1(3), 171-188.
Grove S J. (2002c) Tree basal area and dead wood as surrogate indicators of saproxylic insect faunal integrity: a case study from the Australian lowland tropics, Ecological Indicators, Vol. 1(3), 171-188.
Grove S J. (2002d) The influence of forest management history on the integrity of the saproxylic beetle fauna in an Australian lowland tropical rainforest, Biological Conservation, Vol. 104(2), 149-171.
Gustafson E J. and Gardner R H. (1996) The effect of landscape heterogeneity on the probability of patch colonisation, Ecology, Vol. 77(1), 94-107.
Hammond H J., Langor D W. and Spence J R. (2001) Early colonization of Populus wood by saproxylic beetles (Coleoptera), Canadian Journal of Forest Research, Vol. 31(7), 1175-1183.
Hammond H J., Langor D W. and Spence J R. (2004) Saproxylic beetles (Coleoptera) using Populus in boreal aspen stand of western Canada: spatiotemporal variation and conservation of assemblages, Canadian Journal of Forest Research, Vol. 34(1), 1-19.
Hannah L., Carr J L. and Lankerani A. (1995) Human disturbance and natural habitat: a biome level analysis of a global dataset, Biodiversity Conservation, Vol. 2, 128-155.
Hedgren O. and Weslien J. (2008) Detecting rare species with random or subjective sampling: a case study of red-listed saproxylic beetles in boreal Sweden, Conservation Biology, Vol. 22(1), 212-215.
Hedin J., Ranius T., Nilsson S G. and Smith H G. (2008) Restricted dispersal in a flying beetle assessed by telemetry, Biodiversity and Conservation, Vol. 17(3), 675-684.
Herzog F. (1998) Streuobst: a traditional agroforestry system as a model for agroforestry development in temperate Europe, Agroforestry Systems, Vol. 42(1), 61-80.
Hindmarch T D. and Reid M L. (2001) Thinning of mature lodgepole pine stands increases scolytid bark beetle abundance and diversity, Canadian Journal of Forest Research, Vol. 31(9), 1502-1512.
Hjätlén J., Johansson T., Alinvi O., Danell K., Ball J P., Petterson R., Gibb H. and Hilszczański J. (2007) The importance of substrate type, shading and scorching for the attractiveness of dead wood to saproxylic beetles, Basic and Applied Ecology, Vol. 8(2), 364-376.
Holland J D., Bert D G. and Fahrig L. (2004) Determining the spatial scale of species’ response to habitat, BioScience, Vol. 54(3), 227-233.
Holland J D., Fahrig L. and Cappuccino N. (2005) Body size affects the spatial scale of habitat-beetle interactions, Oikos, Vol. 110, 101-108.
Hopkins A J M., Harrison K S., Grove S J., Wardlaw T J. and Mohammed C L. (2005) Wood-decay fungi and saproxylic beetles associated with living Eucalyptus obliqua trees: early results from studies at the Warra LTER Site, Tasmania, Tasforests, Vol. 16, 111- 126.
Hopkins J J. and Kirby K J. (2007) Ecological change in British broadleaved woodland since 1947, Ibis, Vol. 149(s2), 29-40.
Horák J., Vávrová E. and Chobot K. (2010) Habitat preferences influencing populations, distribution and conservation of the endangered saproxylic beetle Cucujus cinnaberinus (Coleoptera: Cucujidae) at the landscape level, European Journal for Entomology, Vol. 107(1), 81-88.
Horák J., Chumanová E. and Hilszański J. (2012) Saproxylic beetle thrives on the openness in management: a case study on the ecological requirements of Cucujus cinnaberinus from Central Europe, Insect Conservation and Diversity, Vol. 5(6), 403-413.
Horák J. and Rébl K. (2013) The species richness of click beetles in ancient pasture benefits from a high level of sun exposure, Journal of Insect Conservation, Vol. 17, 307-318.
Hottola J. and Siitonen J. (2008) Significance of woodland key habitats for polypore diversity and red-listed species in boreal forests, Biodiversity and Conservation, Vol. 17(11), 2559-2557.
Hultberg T., Brunet J., Broström A. and Lindbladh M. (2010) Forest in a cultural landscape – the vegetation history of Torup in southernmost Sweden, Ecological Bulletins, Vol. 53, 141-153.
Hurt L. and Burrough A. (2009) Nobel Chafer survey 2009, Brockhampton Estate, Herefordshire, PTES, London.
Irmler U., Arp H. and Nötzold R. (2010) Species richness of saproxylic beeltes in woodlands affected by dispersion ability of species, age and stand size, Journal of Insect Ecology, Vol. 14, 227-235.
Jacobs J M., Spence J R. and Langor D W. (2007) Influence of boreal forest succession and dead wood qualities on saproxylic beetles, Agricultural and Forest Entomology, Vol. 9(1), 3-16.
Jansson N. (2009) Habitat requirements and preservation of the beetle assemblages associated with hollow oaks, Doctoral Thesis: Department of Physics, Chemistry and Biology, Linköping University, Linköping, Sweden.
Jansson N., Ranius T., Larsson A. and Milberg P. (2009) Boxes mimicking tree hollows can help conservation of saproxylic beetles, Biodiversity Conservation, Vol. 18(14), 3891-3908.
Jeanneret Ph., Schüpbach B. and Luka H. (2003) Quantifying the impact of landscape and habitat features on biodiveristy in cultivated landscapes, Agriculture, Ecosystems and Environment, Vol. 98, 311-320.
Johansson T., Hjältén J., Gibb H., Hilszczanski J., Stenlid J., Ball J P., Alinvi O. and Danell K. (2007) Variable response of different functional groups of saproxylic beetles to substrate manipulation and forest management: Implications for conservation strategies, Forest Ecology and Management, Vol. 242(2), 496-510.
Joint Nature Conservation Committee (2007) Conserving Biodiversity – the UK Approach, JNCC, Peterborough.
Joint Nature Conservation Committee (2010) UK priority species pages – version 2 – Gnorimus nobilis, JNCC, Peterborough.
Jonsell M., Weslien J. and Ehnström B. (1998) Substrate requirements of red-listed saproxylic invertebrates in Sweden, Biodiversity and Conservation, Vol. 7, 749-764.
Jonsell M., Nordlander G. and Ehnström B. (2001) Substrate associations of insects breeding in fruiting bodies of wood-decaying fungi, Ecological Bulletins, Vol. 49, 173-194.
Jonsell M. and Nordlander G. (2002) Insects in polypore fungi as indicator species: a comparison between forest sites differing in amounts and continuity of dead wood, Forest Ecology and Management, Vol. 157(1), 101-118.
Jonsell M. and Weslien J. (2003) Felled or standing retained wood – it makes a difference for saproxylic beetles, Forest Ecology and Management, Vol. 175(1-3), 425-435.
Jonsell M., Schroeder M. and Weslien J. (2005) Saproxylic beetles in high stumps of spruce: Fungal flora important for determining the species composition, Scandinavian Journal of Forest Research, Vol. 20(1), 54-62.
Jonsson B G., Kruys N. And Ranius T. (2005) Ecology of species living on dead wood – lessons for dead wood management, Silva Fennica, Vol. 39(2), 289-309.
Jørgen K. and Karsten T. (1994) A new method for measuring tree height in tropical rain forest, Journal of Vegetation Science, Vol. 5, 139-140.
Jukes M R., Ferris R. and Peace A J. (2002) The influence of stand structure and composition on diversity of canopy Coleoptera in coniferous plantations in Britain, Forest Ecology and Management, Vol. 163(1-3), 27-41.
Kaila L., Martikainen P., Punttila P. and Yakovlev E. (1994) Saproxylic beetles (Coleoptera) on dead birch trunks decayed by different polypore species, Annals of Zoology Fennici, Vol. 31, 97-107.
Kindlmann P., Aviron S. and Burel F. (2005) When is landscape matrix important for determining animal fluxes between resource patches?, Ecological Complexity 2, 150-158.
Kirby K J., Thomas R C., Key R S., McLean I F G. and Hodgetts N. (1995) Pasture-woodland and its conservation in Britain, Biological Journal of the Linnean Society, Vol. 56(s1), 135-153.
Kouki J., Hyvärinen E., Lappalainen H., Martikainen P. and Simila M. (2012) Landscape context affects the success of habitat restoration: large-scale colonization patterns of saproxylic and fire-associated species in boreal forests, Diversity and Distributions, Vol. 18, 348-355.
Koziell S P. (2000) A tale of two apples, Resurgence, Vol. 198, 44-45.
Krikken J. (1984) A new key to the suprageneric taxa in the beetle family Cetoniidae, with annotated lists of the known genera, Zoologische Verhandelingen, Vol. 210(1), 1-75.
Lachat T., Nagel P., Cakpo Y., Attignon S., Goergen G., Sinsin B. and Peveling R. (2006) Dead wood and saproxylic beetle assemblages in a semi-deciduous forest in Southern Benin, Forest and Ecology Management, Vol. 225(1-3), 27-38.
Lachat T., Wermelinger B., Gossner M M., Buβler H., Isacsson G. and Müller J. (2012) Saproxylic beetles as indicator species foe dead-wood amount and temperature in European beech forests, Ecological Indicators, Vol. 23, 323-331.
Lang G A. (2001) Intensive Sweet Cherry Orchard Systems – Rootstocks, Vigor, Precocity, Productivity and Management, The Compact Fruit Tree, Vol. 34(1), 23-26.
Larrieu L., Cabanettes A. and Delarue A. (2012) Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees, European Journal of Forest Research, Vol. 131(3), 773-786.
Lassauce A., Paillet Y., Jactel H. and Bouget C. (2011) Deadwood as a surrogate for forest biodivsersity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms, Ecological Indicators, Vol. 11(5), 1027-1039.
Legendre P. (1993) Spatial autocorrelation: Trouble or New Paradigm?, Ecology, Vol. 74(6), 1659-1673.
Levins R. (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control, Bulletin of the Entomological Society of America, Vol. 15, 237-240.
Lindhe A., Lindelöw A. and Åsenblad N. (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter, Biodiversity and Conservation, Vol. 14, 3033-3053.
Lindo Z. Winchester N N. and Didham R K. (2008) Nester patterns of community assembly in the colonisation of artificial canopy habitats by oribatid mites, Oikos, Vol. 117(12), 1856-1864.
Lindo Z. and Winchester N N. (2009) Spatial and environmental factors contributing to patterns in arboreal and terrestrial oribatid mite diversity across spatial scales, Oecologia, Vol. 160(4), 817-825.
Lizeé M-H., Manel S., Mauffrey J-F., Tatoni T. and Deschamps-Cottin M. (2012) Matrix configuration and patch isolation influences override the species-area relationship for urban butterfly communities, Landscape Ecology, Vol. 27(2), 159-169.
Löfman S. and Kouki J. (2001) Fifty years of landscape transformation in managed forests of southern Finland, Scandinavian Journal of Forest Research, Vol. 16(1), 44-53.
Lush M., Robertson H J., Alexander K N A., Giavarni V., Hewins E., Mellings J., Stevenson C R., Storey M. and Whitehead P F. (2009) Biodiversity studies of six traditional orchards in England, Natural England Research Reports, No. 025.
MacDonald D W., King C M. and Strachan R. (2007) Introduced species and the line between biodiversity conservation and naturalistic eugenics, in Macdonald D. and Service K. (eds.), Key Topics in Conservation Biology, Blackwell, Malden, 186-205.
Mackey B G. and Lindenmayer D B. (2001) Towards a hierarchical framework for modelling the spatial distribution of animals, Journal of Biogeography, Vol. 28(9), 1147-1166.
Mäkipää R. and Linkosalo T. (2011) A non-destructive field method for measuring wood density of decaying logs, Silva Fennica, Vol. 45(5). 1135-1142.
Mankin R W., Mizrach A., Hetzroni A., Levsky S., Nakache Y. and Soroker V. (2008) Temporal and spectral features of sounds of wood-boring beetle larvae: identifiable patterns of activity enable improved discrimination from background noise, Florida Entomologist, Vol. 91(2), 241-248.
Marshall D. (2008) Herefordshire orchard community evaluation project, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 96-100.
Martikainen P., Siitonen J., Punttila P., Kaila L. and Rauh J. (2000) Species richness of Coleoptera in mature managed and old-growth boreal forests in southern Finland, Biological Conservation, Vol. 94(2), 199-209.
Martikainen P. (2001) Conservation of threatened saproxylic beetles: significance of retained aspen Populus tremula on clearcut areas, Ecological Bulletins, Vol. 49, 205-218.
Martikainen P. and Kouki J. (2002) Sampling the rarest: threatened beetles in boreal forest biodiversity inventories, Biodiversity and Conservation, Vol. 12(9), 1815-1831.
Mattson K G., Swank W T. and Waide J B. (1987) Decomposition of woody debris in a regenerating, clear-cut forest in the Southern Appalachians, Canadian Journal of Forest Research, Vol. 17(7), 712-721.
Mazzerolle M J. and Villard M. (1999) Patch characteristics and landscape context as predictors of species presence and abundance: a review, Ecoscience, Vol. 6(1), 117-124.
McGeoch M A., Schroeder M., Ekbom B. and Larsson S. (2007) Saproxylic beetle diversity in a managed boreal forest: importance of stand characteristics and forestry conservation measures, Diversity and Distributions, Vol. 13(4), 418-429.
Meggs J M., Munks S A. and Corkrey R. (2003) The distribution and habitat characteristics of a threatened lucanid beetle Hoplogonus simsoni in north-east Tasmania, Pacific Conservation Biology, Vol. 9(3), 172-186.
Merwin I A. and Stiles W C. (1994) Orchard groundcover management impacts on apple tree growth and yield, and nutrient availability and uptake, Journal of the American Society for Horticultural Science, Vol. 199(2), 209-215.
Michel A K. and Winter S. (2009) Tree microhabitat structures as indicators of biodiversity in Douglas-fir forests of different stand ages and management histories in the Pacific Northwest, U.S.A., Forest Ecology and Management, Vol. 257(6), 1453-1464.
Molina-Montenegro M A., Badano E I. and Cavieres L A. (2006) Cushion plants as microclimatic shelters for two ladybird species in alpine zone of central Chile, Arctic, Antarctic and Alpine Research, Vol. 38(2), 224-227.
Müller J., Buβler H. And Kneib T. (2008) Saproxylic beetle assemblages related to silvicultural management intensity and stand structures in a beech forest in Southern Germany, Journal of Insect Conservation, Vol. 12, 107-124.
Müller J. and Brandl R. (2009) Assessing biodiversity by remote sensing in mountainous terrain: the potential of LiDAR to predict forest beetle assemblages, Journal of Applied Ecology, Vol. 46(4), 897-905.
Müller J. and Bütler R. (2010) A review of habitat thresholds for dead wood: a baseline for management recommendations in European forests, European Journal of Forest Research, Vol. 129(6), 981-992.
Müller J. and Goβner M M. (2010) Three-dimensional partitioning of diversity informs state-wide strategies for the conservation of saproxylic beetles, Biological Conservation, Vol. 143(3), 625-633.
Murcia C. (1995) Edge effects in fragmented forests: implications for conservation, Trends in Ecology and Evolution, Vol. 10(2), 58-62.
Murphy H T. and Lovett-Doust J. (2004) Context and connectivity in plant metapopulations and landscape mosaics: does the matrix matter?, Oikos, Vol. 105(1), 3-14.
Nagendra H., Lucas R., Pradinho Honrado J., Jongman R H G., Tarantino C., Adamo M. and Mairota P. (2013) Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat conditions, species diveristy and threats, Ecological Indicators, Vol. 33(1), 45-59.
Natural England (2012) Nature on the Map, http://www.natureonthemap.naturalengland .org.uk/map.aspx?m=bap, accessed: 23/03/2013.
Niemelä J. (1997) Invertebrates and Boreal forest management, Conservation Biology, Vol. 11(3), 601-610.
Nieto A. and Alexander K N A. (2010) European Red List of Saproxylic Beetles, Publication Office of the EU, Luxembourg.
Nilsson S G. (1997) Forests in the temperate-boreal transition: natural and man-made features, Ecological Bulletins, Vol. 46, 61-71.
Nilsson S G. and Baranowski R. (1997) Habitat predictability and the occurrence of wood beetles in old-growth beech forests, Ecography, Vol. 20, 491-498.
Nilsson S G., Hedin J. and Niklasson M. (2001) Biodiversity and its assessment in boreal and nemoral forests, Scandinavian Journal of Forest Research, Vol. 16 (s3), 10-26.
Nilsson S G., Niklasson M., Hedin J., Aronsson G., Gutowski J M., Linder P., Ljungberg H., Mikusinski G., Ranius T. (2002) Densities of large living and dead trees in old-growth temperate and boreal forests, Forest Ecology and Management, Vol. 161(1), 189-204.
Ódor P., Heilmann-Clausen J., Christensen M., Aude E., van Dort K W., Piltaver A., Siller I., Veerkamp M T., Walleyn R., Standovár T., van Hees A F M., Kosec J., Matočec N., Kraigher H. and Grebenc T. (2006) Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe, Biological Conservation, Vol. 131(1), 58-71.
Økland B., Bakke A., Hågvar S. And Kvamme T. (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway, Biodiversity and Conservation, Vol. 5, 75-100.
Oleska A., Urlich W. and Gawroński R. (2007) Host tree preferences of Hermit beetles (Osmoderma eremit Scop., Coleoptera: Scarabaeidae) in a network of rural avenues in Poland, Polish Journal of Ecology, Vol. 55(2), 315-323.
Orłowski G. and Nowak L. (2007) The importance of marginal habitats for the conservation of old trees in agricultural landscapes, Landscape and Urban Planning, Vol. 79(1), 77-83.
Osborne P J. (1974) An insect assemblage of early Flandrian age from Lea Marston, Warwickshire, and its bearing on the contemporary climate and ecology, Quaternary Research, Vol. 4(4), 471-486.
Östman Ö., Ekbom B. and Bengtsson J. (2001) Landscape heterogeneity and farming practice influence biological control, Basic and Applied Ecology, Vol. 2(4), 365-371.
Paracchini M L., Terres J M., Petersen J E. and Hoogeveen Y B. (2007) High nature value farmland and traditional agricultural landscapes, in Pedroli B., Van Doorn A., De Blust G., Paracchini M L., Wascher D. and Bunce F. (eds.), Europe’s living landscapes. Essays on exploring our identity in the countryside, Landscape Europe, Wageningen, 22-34.
Pentillä R., Siitonen J. and Kuusinen M. (2004) Polypore diversity in managed and old growth boreal Picea abies forests in southern Finland, Biological Conservation, Vol. 117(3), 271-283.
People’s Trust for Endangered Species (2008a) Noble Chafer Fact sheet, PTES, London.
People’s Trust for Endangered Species (2008b) Noble Chafer (Gnorimus nobilis), PTES, London.
Petit C C. and Lambin E F. (2002) Impact of data integration technique on historical land-use/land-cover change: Comparing historical maps with remote sensing data in the Belgian Ardennes, Landscape Ecology, Vol. 17(2), 117-132.
Philpott S M., Arendt W J., Armbrecht I., Bichier P., Diestch T V., Gordon C., Greenberg R., Perfecto I., Reynoso-Santos R., Soto-Pinto L., Tejeda-Cruz C., Williams-Linera G., Valenzuela J. and Zolotoff J M. (2008) Biodiversity Loss in Latin American Coffee Landscapes: Review of the Evidence on Ants, Birds, and Trees, Conservation Biology, Vol. 22(5), 1093-1105.
Pimm S L., Russell G J., Gittleman J L. and Brooks T M. (1995) The Future of Biodiversity, Science, Vol. 269(5222), 347-350.
Plieninger T., Höchtl F. and Spek T. (2006) Traditional land-use and nature conservation in European rural landscapes, Environmental Science and Policy, Vol. 9(4), 317-321.
Pyle C. and Brown M M. (1999) Heterogeneity of wood decay classes within hardwood logs, Forest Ecology and Management, Vol. 114(2-3), 253-259.
Ranius T. and Nilsson S G. (1997) Habitat of Osmoderma eremita Scop. (Coleoptera: Scarabaeidae), a beetle living in hollow trees, Journal of Insect Conservation, Vol. 1(4), 193-204.
Ranius T. (2000) Minimum viable metapopulation size of a beetle, Osmoderma eremita, living in tree hollows, Animal Conservation, Vol. 391), 37-43.
Ranius T. and Jansson N. (2000) The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associate with old oaks, Biological Conservation, Vol. 95(1), 85-94.
Ranius T. and Wilander P. (2000) Occurrence of Larca lata H.J. Hansen (Pseudoscorpionida: Garypidae) and Allochernes wideri C.L. Koch (Pseudoscorpionida: Chernetidae) in tree hollows in relation to habitat quality and density, Journal of Insect Conservation, Vol. 4, 23-31.
Ranius T. (2001) Constancy and asynchrony of Osmoderma eremita populations in tree hollows, Oecologia, Vol. 126(2), 208-215.
Ranius T. (2002a) Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden, Biological Conservation, Vol. 103, 85-91.
Ranius T. (2002b) Osmoderma eremita as an indicator of species richness of beetles in tree hollows, Biodiversity and Conservation, Vol. 11(5), 931-941.
Ranius T. and Jannson N. (2002) A comparison of three methods to survey saproxylic beetles in hollow oaks, Biodiversity and Conservation, Vol. 11(10), 1759-1771.
Ranius T. (2003) Habitat fragmentation affects beetles and pseudoscorpions living in hollow oaks in Sweden, Proceedings of the second pan-European conference on Saproxylic Beetles, PTES, London.
Ranius T. (2006) Measuring the dispersal of saproxylic insects: a key characteristics of their conservation, Population Ecology, Vol. 48, 177-188.
Ranius T. and Fahrig L. (2006) Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds, Scandinavian Journal of Forest Research, Vol. 21(3), 201-208.
Ranius T. (2007) Extinction risks in metapopulations of a beetle inhabiting hollow trees predicted from time series, Ecography, Vol. 30(5), 716-726.
Ranius T., Niklasson M. and Berg N. (2009a) Development of tree hollows in pedunculate oak (Quercus robur), Forest Ecology and Management< Vol. 257, 303-310.
Ranius T., Svensson G P., Berg N., Niklasson M. and Larsson M C. (2009b) The successional change of hollow oaks affects their suitability for an inhabiting beetle, Osmoderma eremita, Annals of Zoology Fennici, Vol. 46(3), 205-216.
Ranius T. and Roberge J M. (2011) Effects of intensified forestry on the landscape-scale extinction risk of dead wood dependent species, Biodiversity Conservation, Vol. 20(13), 2867-2882.
Read H. (2000) Veteran trees: A guide to good management, English Nature, Peterborough.
Regnery B., Paillet Y., Couvet D. and Kerbiriou C. (2013) Which factors influence the occurrence and density of tree microhabitats in Mediterranean oak forests?, Forest and Ecology Management, Vol. 295, 118-125.
Renault D., Vernon P. and Vannier G. (2005) Critical thermal maximum and body water loss in first instar larvae of three Cetoniidae species (Coleoptera), Journal of Thermal Biology, Vol. 30(8), 611-617.
Robertson H J. and Wedge C. (2008) Traditional Orchards and the UK Biodiversity Action Plan, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 109-118.
Robinson G M. (1983) The evolution of the horticultural industry in the Vale of Evesham, Scottish Geographical Magazine, Vol. 99(2), 89-100.
Robinson R. and Sutherland W (2002) Post-war changes in arable farming and biodiversity in Great Britain, Journal of Applied Ecology, Vol. 39, 157–176.
Rotheray E L. (2013) Differences in ecomorphology and microhabitat use of four saproxylic larvae (Diptera, Syrphidae) in Scots pine stump rot holes, Ecological Entomology, Vol. 38(3), 219-229.
Rounsevell M D A., Annetts J E., Audsley E., Mayr T. and Reginster I. (2003) Modelling the spatial distribution of agricultural land use at the regional scale, Agriculture, Ecosystems and Environment, Vol. 95., 465-479.
Rukke B A. and Midtgaard F. (1998) The importance of scale and spatial variables for the fungivorous beetle Bolitophagus reticulatus (Coleoptera, Tenebrionidae) in a fragmented forest landscape, Ecography, Vol. 21(6), 561-572.
Rukke B A. (2000) Effects of habitat fragmentation: increased isolation and reduced habitat size reduces the incidence of dead wood fungi beetles in a fragmented forest landscape, Ecography, Vol. 23(4), 492-502.
Russo D., Cistrone L. and Garonna A P. (2011) Habitat selection by the highly endangered longhorn beetle Rosalia alpine in Southern Europe: a multiple spatial scale assessment, Journal of Insect Conservation, Vol. 15(5), 685-693.
Sadler J P., Small E C., Fiszpan H., Teffer M G. and Niemelä (2006) Investigating environmental variation and landscape characteristics of an urban-rural gradient using woodland carabid assemblages, Journal of Biogeography, Vol. 33(6), 1126-1138.
Sahlin E. and Schroeder L M. (2010) Importance of habitat patch size for occupancy and density of aspen-associated saproxylic beetles, Biodiversity Conservation, Vol. 19(5), 1325-1339.
Schiegg K. (2001) Saproxylic insect diversity of beech: limbs are richer than trunks, Forest Ecology and Management, Vol. 149(1-3), 295-304.
Schroeder L M., Ranius T., Ekbom M. and Larsson S. (2007) Spatial occurrence of a habitat-tracking saproxylic beetle inhabiting a managed forest landscape, Ecological Applications, Vol. 17(3), 900-909.
Schweiger O., Maelfait J P., Van Wingerden W K R E., Hendrickx F., Billeter R., Speelmans M., Augenstein I., Aukema B., Aviron S., Bailey D., Bukacek R., Burel F., Diekötter T., Dirksen J., Frenzel M., Herzog F., Liira J., Roubalova M. and Bugter R. (2005) Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales, Journal of Applied Ecology, Vol. 42(6), 1129-1139.
Sebek P., Altman J., Platek M. and Cizek L. (2013) Is active management the key to the conservation of saproxylic Biodiversity? Pollarding promotes the formation of tree hollows, PloS one, Vol. 8(3), e60456, 1-6.
Sharma A. and Panigrahy S. (2007) Apple orchard characterization using remote sensing and GIS in Shimla district of Himachal Pradesh, The Proceedings of the Remote Sensing and Photogrammetry Annual Conference, RSPS, Newcastle,
Sharples L. (2007) Apples, cider and celebration, in Hall C M. and Sharples L. (eds.), Food and wine festivals and events around the world, Butterworth-Heinemann, Oxford, 133-145.
Siitonen J. (1994) Decaying wood and saproxylic Coleoptera in two old spruce forests: a comparison based on two sampling methods, Annals of Zoology Fennici, Vol. 31, 89-95.
Siitonen J. (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example, Ecological Bulletins, Vol. 49, 11-41.
Siitonen J. and Saaristo L. (2000) Habitat requirements and conservation of Pytho kolwensis, a beetle species of old-growth boreal forest, Biological Conservation, Vol. 94(2), 211-220.
Similä M., Kouki J., Mönkkönen M. and Sippola A L. (2002) Beetle species richness along the forest productivity gradient in northern Finland, Ecography, Vol. 25(1), 42-52.
Similä M., Kouki J. and Martikainen P. (2003) Saproxylic beetles in managed and seminatural Scots pine forests: quality of dead wood matters, Forest Ecology and Management, Vol. 174(1-3), 365-381.
Similä M., Kouki J., Mönkkönen M. and Sippola A L. and Huhta E. (2006) Co-variation and indicators of species diversity: Can richness of forest-dwelling species be predicted in northern boreal forests?, Ecological Indicators, Vol. 6(4), 686-700.
Sippola A L., Siitonen J. and Punttila P. (2002) Beetle diversity in timberline forests: a comparison between old-growth and regeneration areas in Finnish Lapland, Annals of Zoology Fennici, Vol. 39, 69-86.
Sirami C., Jay-Robert P., Brustel H., Valladares L., Le Guilloux S., Martin J L., (2008) Saproxylic beetle assemblages of old Holm-oak trees in the Mediterranean region: role of a keystone structure in a changing heterogeneous landscape, Revue d Ecologie (la Terre et La Vie), Vol. 63, 93–106.
Smith M N. (2003) Saproxylic beetles in Britain, an overview of the status and distribution of four Biodiversity Action Plan species, Proceedings of the second pan-European conference on Saproxylic Beetles, PTES, London.
Speight M C D. (1989) Saproxylic invertebrates and their conservation, Council of Europe, Strasbourg.
Stoate C., Báldi A., Beja P., Boatman N D., Herzon I., van Doorn A., de Snoo G R., Rakosy L. and Ramwell C. (2009) Ecological impacts of early 21st century agricultural change in Europe – A review, Journal of Environmental Management, Vol. 91(1), 22-46.
Stoner K J L. and Joern A. (2004) Landscape vs. Local habitat scale influences to insect communities from tallgrass prairie remnants, Ecological Applications, Vol. 14(5), 1306-1320.
Sverdrup-Thygeson A. (2001) Can ‘continuity indicator species’ predict species richness or red-listed species of saproxylic beetles?, Biodiversity and Conservation, Vol. 10, 815-832.
Szacki J. (1999) Spatially structured populations: how much do they match the classic metapopulation concept?, Landscape Ecology, Vol. 14(4), 369-379.
Tahvanainen J O. (1972) Phenology and microhabitat selection of some flea beetles (Coleoptera: Chrysomelidae) on wild and cultivated crucifers in central New York, Insect Systematics and Evolution, Vol. 3(2), 120-138.
Tauzin P. (2000) Le genre Aleurostictus Kirby, 1827. Contribution à sa connaisance et précision sur la distribution des espèces (Coleoptera, Cetoniidae, Trichiinae, Trichiini), L’Entomologiste, Vol. 56(6), 231-281.
Tischendorf L. and Fahrig L. (2000) On the use and measurement of landscape connectivity, Oikos, Vol. 90(1), 7-19.
Tikkanen O-P., Martikainen P., Hyvärinen E., Junninen K. and Kouki J. (2006) Red-listed boreal forest species of Finland: associations with forest structure, tree species, and decaying wood, Annals of Zoology Fennici, Vol. 43, 373-383.
Tikkanen O-P., Heinone T., Kouki J. and Matero K. (2007) Habitat suitability models for saproxylic red-listed boreal forest species in long-term matrix management: Cost-efective measures for multi-species conservation, Biological Conservation, Vol. 140(3-4), 359-372.
Timmer L W., Sandler H A., Graham J H. and Zitko S E. (1988) Sampling citrus orchards in Florida to estimate populations of Phytophthora parasitica, Phytopathology, Vol. 78(7), 940-944.
Tscharntke T., Steffan-Dewenter I., Kruess A. and Thies C. (2002) Characteristics of insect populations on habitat fragments: A mini review, Ecological Research, Vol. 17(2), 229-239.
Tscharntke T., Klein A M., Kruess A., Steffan-Dewenter I. and Thies C. (2005) Landscape perspectives on agricultural intensification and biodiversity – ecosystem service management, Ecology Letters, Vol. 8(8), 857-874.
Turner W., Spector S., Gardiner N., Fladeland M., Sterling E. and Steininger M. (2003) Remote sensing for biodiversity science and conservation, Trends in Ecology and Evolution, Vol. 18(6), 306-314.
Ulyshen M D. and Hanula J L. (2009) Habitat associations of saproxylic beetles in the southeastern United States: A comparison of forest types, tree species and wood postures, Forest Ecology and Management, Vol. 257(2), 653-664.
Vernon P. and Vannier G. (2001) Freezing susceptibility and freezing tolerance in Palaeartic Cetoniidae (Coleoptera), Canadian Journal of Zoology, Vol. 79(1), 67-74.
Wagner H H. and Fortin M-J. (2005) Spatial analysis of landscapes: concepts and statistics, Ecology, Vol. 86(8), 1975-1987.
Warner T A. and Steinmaus K. (2005) Spatial classification of orchards and vineyards with high spatial resolution panchromatic imagery, Photogrammetric Engineering and Remote Sensing, Vol. 71(2), 179-187.
Warren M S. and Key R S. (1991) Woodlands: Past, Present and Potential for Insects, in Collins N M. and Thomas J A. (eds.), The Conservation of Insects and their Habitats, Harcourt Brace Jovanovich, London, 155-203.
Webb A., Buddle C M., Drapeau P. and Saint-Germain M. (2008) Use of remnant boreal forest habitats by saproxylic beetle assemblages in even-aged managed landscapes, Biological Conservation, Vol. 141(3), 815-826.
Weedon J T., Cornwell W K., Cornelissen J H C., Zanne A E., Wirth C. and Coomes D A. (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species?, Ecology Letters, Vol. 12, 45-56.
Wermelinger B., Flückiger P F., Obrist M K. and Duelli P. (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges, Journal of Applied Entomology, Vol. 131(2), 104-114.
Whitehead P F. (1992) Fruit trees, orchards and their rare invertebrates, Entomologist’s Gazette, Vol. 43, 303-304.
Whitehead P F. (1997) Invertebrates of pome and stone fruit orchards in the English Wyre Forest, July 1997, with particular regard to Gnorimus nobilis (L.,1758) (Col., Scarabaeidae), 1-8, Unpublished report for English Nature.
Whitehead P F. (2003) The noble chafer Aleurostictus nobilis (L., 1758)(Col., Scarabaeidae) in Britain, Proceedings of the second pan – European conference on saproxylic beetles, PTES, London.
Whiting (2002) Phylogeny of the holometabolous insect orders: molecular evidence, Zoologica Scripta, Vol. 31(1), 3-15.
Widdicome R. (2008) Traditional orchards: threats and opportunities within the planning system for Herefordshire and Worcestershire, in Rotherham I D. (ed) Orchards and Groves: their history, ecology, culture and archaeology. Landscape Archaeology and Ecology, Vol. 7, 143-155.
Wilson E O. (2002) The Future of Life, Little Brown, London.
With K A. and Crist T O. (1995) Critical threholds in species’ responses to landscape structure, Ecology, Vol. 76(8), 2446-2459.
With K A., Cadaret S J. and Davis C. (1999) Movement responses to patch structure in experimental fractal landscapes, Ecology, Vol. 80, 1340–1353.
Woodman J D., Cooper P D. and Haritos V S. (2007) Effects of temperature and oxygen availability on water loss and carbon dioxide release in two sympatric saproxylic invertebrates, Comparative Biochemistry Part A: Molecular and Integrative Physiology, Vol. 147(2), 514-520.
Worcestershire Biodiversity Partnership (2008) Worcestershire Biodiversity Action Plan 2008: S5 Noble Chafer SAP, Worcestershire Biodiversity Partnership Publications, Worcester.
Wrigley E A. (1985) Urban growth and agricultural change: England and the continent in the early modern, The Journal of Interdisciplinary History, Vol. 15(4), 683-728.
Wright D H. (1983) Species-energy theory – an extension of the species area theory, Oikos, Vol. 41, 496-506.
Wu J., Yu X-D., Zhou H-Z. (2009) The saproxylic beetle assemblage associated with different host trees in Southwest China, Insect Science, Vol. 15(3), 251-261.
Wünsch A. and Hormaza J I. (2002) Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers, Euphytica, Vol. 125(1), 59-67.
Yee M., Growe S J., Richardson A. and Mohammed C L. (2006) Brown rot in inner heartwood: why large logs support characteristic saproxylic beetle assemblages of conservation concern, in Grove S J. and Hanula J L. (eds.), Insect biodiversity and dead wood: proceedings of a symposium for the 22nd International Congress of Entomology, U.S. Department of Agriculture, Forest Service, Southern Research Station, Asheville, 42-56.
Zdenĕk B P., Turčáni M. and Horák J. (2012) Sharing the same space: foraging behaviour of saproxylic beetles in relation to dietary components of morphologically similar larvae, Ecological Entomology, Vol. 37, 117-123.
Zuur A F., Ieno E N. And Elphick C S. (2010) A protocol for data exploration to avoid common statistical problems, Methods in Ecology and Evolution, Vol. 1(1), 3-14.
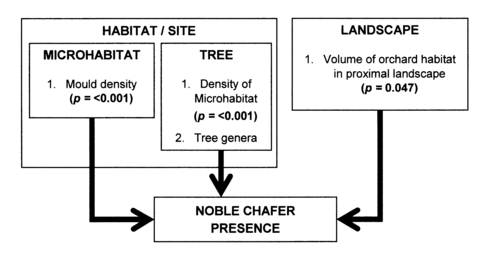
01. Factors influencing the presence of Gnorimus nobilis.
Worcestershire Record | 37 (November 2014) page: 54-66 | Worcestershire Biological Records Centre & Worcestershire Recorders